US Pharm. 2017;42(3):28-32.
ABSTRACT: Prescription opioid use has significantly increased globally in the past two decades and has led to an increased number of patients who have become tolerant to opioids. Analgesia in this patient population poses a challenge, and there is a risk of undertreatment. In addition to providing effective analgesia, it is important to prevent withdrawal symptoms and address any related psychosocial issues. Therefore, a multidisciplinary and multimodal approach in pain management is necessary to provide effective analgesia in this patient population.
Prescription opioids are some of the most commonly prescribed pain medications in the United States, and they are usually the drugs of choice for managing moderate-to-severe pain. The number of opioids prescribed in the U.S. skyrocketed from 76 million in 1991 to 216 million in 2012, with one-half of opioid sales accounting for treatment of chronic noncancer pain.1,2
The FDA defines a patient as opioid tolerant if for at least 1 week he or she has been receiving oral morphine 60 mg/day; transdermal fentanyl 25 mcg/hour; oral oxycodone 30 mg/day; oral hydromorphone 8 mg/day; oral oxymorphone 25 mg/day; or an equianalgesic dose of any other opioid.3 Opioid tolerance implies a lesser susceptibility to the effects of opioids—both therapeutic and adverse—and it may develop in individuals with long-term use of opioids.3-6 Patients who are prescribed opioids for management of cancer pain or chronic noncancer pain or who have an opioid addiction may become opioid tolerant.5,6
Acute pain may present postoperatively because of the traumatic surgical injury, or in other acute conditions. Acute pain in patients with opioid tolerance makes pain management a challenge, and perhaps one of the greatest risks associated with pain management in this population is the risk of undertreatment due to stigma and bias. Further, data on pain management in this patient population are limited.7 This review focuses primarily on acute pain management in opioid-tolerant patients, including those with chronic opioid use for chronic noncancer pain and those who abuse opioids.
PATHOPHYSIOLOGY
The sensation of pain occurs via nociception, a process of communication between the site of tissue damage and the central nervous system (CNS).8,9 A noxious stimulus (mechanical, thermal, or chemical) activates nociceptors, or free nerve endings (A-delta and C fibers), in the peripheral nerves.9 This stimulus is then transduced into a sensor potential that, if high enough, triggers action potential that is transmitted down the axon to nerves located in the dorsal horn of the spinal cord or brainstem.9 The nerve impulses in the dorsal horn may then travel up the ascending pathway to the thalamocortical system, where perception—including information on location, intensity, and duration—occurs.8,9 Pain modulation takes place via the descending pathway from the brain to the neurons in the dorsal horn of the spinal cord.8,9
Neuropathic pain differs from nociceptive pain in that neuropathic pain results from abnormal processing of nerve impulses in the peripheral nervous system and CNS, whereas nociceptive pain results from noxious stimulation of free nerve endings in the tissue.8,9
It is theorized that, with chronic opioid exposure, extensive adaptations occur at the cellular and synaptic levels, leading to opioid tolerance.4 Opioid tolerance is thought to develop from the desensitization, internalization, and downregulation of opioid receptors.4 This phenomenon should not be confused with opioid addiction, in which a physical dependence results from receptor counteradaptation in conjunction with a drug-seeking behavior.4 Physical dependence is a state of adaptation that is manifested by drug-specific withdrawal syndrome upon abrupt change or discontinuation of the drug.4
A condition that overlaps with opioid tolerance is opioid-induced hyperalgesia (OIH). However, the difference between them is that in opioid tolerance, an increased amount of opioids is necessary to relieve the pain, whereas in OIH, the same amount of opioid causes paradoxically worse pain.5 It is important for both clinicians and patients to understand the difference between opioid tolerance and OIH.
CONSEQUENCES OF UNRELIEVED PAIN
Pain is subjective, which makes it difficult to assess the degree of severity. Generally, acute pain is a multidimensional experience, usually resulting from trauma, that lasts no longer than 3 to 6 months, but it has the potential to become more complex, both physiologically and psychologically.10,11 Literature suggests that undertreatment of acute pain may lead to decreased responsiveness to opioid analgesics, thereby making subsequent pain control more difficult.
Uncontrolled pain affects various systems, including the CNS and the cardiovascular, pulmonary, gastrointestinal, renal, immunologic, and muscular systems. Additionally, overall recovery is significantly affected, and progression to chronic pain (pain that is persistent in nature, lasting longer than 6 months) may result.12 The presence of two or more comorbid conditions further complicates pain, and parallels can be drawn between chronic pain and other chronic conditions, such as insomnia, cognitive decline, and depression.
Pain and depression are two of today’s most critical public-health issues. This comorbidity is associated with a greater burden to the patient than for either condition alone.13 Approximately 50% of patients with depression report experiencing pain symptoms.14 In fact, patients with comorbid depression have greater pain, a worse prognosis, and more functional disability.15 When pain is not appropriately treated, postanesthesia care-unit stays are prolonged, hospital discharges are delayed, and unanticipated admissions following ambulatory surgery or readmissions occur.12
TREATMENT GOALS FOR ACUTE PAIN
As outlined in the clinical practice guidelines, goals for acute pain management include 1) early intervention, with frequent regimen adjustments for uncontrolled pain; 2) reduction of pain to an acceptable level determined by the patient; and 3) facilitation of recovery from the underlying disease or injury.16
It is also important to aim for prevention of adverse drug events and opioid-withdrawal symptoms; improved quality of life; and assistance with any social, psychiatric, or behavioral components that may hinder effective pain management.4,5,8 Ultimately, the goal is to achieve a regimen that adequately relieves the patient’s pain without any undue treatment burden, such as harmful side effects.
APPROACH TO PAIN MANAGEMENT
Acute pain management in opioid-tolerant patients often requires a multimodal and multidisciplinary approach; therefore, it is necessary to maintain careful coordination and effective communication between disciplines, as well as between each discipline and the patient.17 Early identification through a careful assessment and history in patients at risk for opioid tolerance is essential for adequate pain-management planning.17,18 Upon identification of such a patient, steps should be taken to formulate an effective treatment plan in the preoperative or similarly acute phase to ensure adequate pain management during the acute period and prevention of withdrawal symptoms or other treatment-related complications.17,18 Similarly, appropriate discharge and transition-of-care planning should be considered.17,18
As with the general patient population, a multimodal pain regimen with a combination of pharmacologic and nonpharmacologic approaches is ideal. Opioids remain the drug of choice for severe pain and are a common option for moderate pain, but multimodal pain management with acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications (TABLE 1) remains the mainstay of effective analgesia. In order to prevent delays in care and the risk of untreated pain, analgesics should be administered on a scheduled basis rather than an as-needed basis.
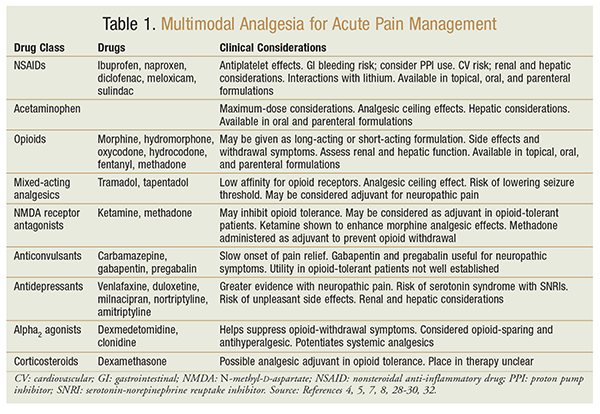
Opioids
Patients with opioid tolerance will likely require more opioids than opioid-naïve patients.4,5 Opioids should be administered in an amount sufficient to achieve analgesia without harmful side effects, such as excessive sedation or respiratory depression.4 Opioid rotation (substitution with a different opioid when one opioid in increasing doses does not provide desired analgesic effect) may be employed when needed in patients with opioid tolerance, as cross-tolerance is uncommon.4,5 The recommended approach for opioid rotation is to initially substitute with one-half to two-thirds equianalgesic opioid and then monitor for safety and effectiveness.4 It should be kept in mind that switching from a long-acting opioid to a short-acting opioid may precipitate withdrawal symptoms in the patient.4
Opioid-related side effects are less common in opioid-tolerant patients; however, if opioid therapy is selected as analgesia of choice in these patients, monitoring for side effects or complications related to opioid therapy remains just as important as with the general population.4,5 The risk of adverse drug events is greater with rapid escalation of opioid therapy, even in opioid tolerance.5 In fact, opioid-tolerant patients are said to be more susceptible to the sedative properties of opioids and should be monitored.5,17
In the selection of opioids as analgesia of choice, patient-controlled analgesia (PCA) offers a convenient method of delivery, as it minimizes the risk of undertreatment, allows self-titration, and negates possible conflicts with nursing staff.4,5 Additionally, a retrospective study found that opioid-tolerant patients who had PCA were less likely to report adverse effects—with the exception of sedation—compared with the opioid-naïve group.19 By nature of their condition, opioid-tolerant patients likely require higher bolus doses with PCA.
Recognizing Acute Withdrawal
Opioid withdrawal can occur in opioid-dependent patients receiving a reduced amount of their usual opioid or using an opioid antagonist. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, lists four criteria for opioid withdrawal: 1) previously heavy and prolonged opioid use ceases or is reduced, or an opioid antagonist is administered after a period of opioid use; 2) at least three signs and symptoms (e.g., dysphoric mood, nausea, vomiting, diarrhea, muscle aches, rhinorrhea, lacrimation, pupillary dilation, piloerection, sweating, yawning, fever, insomnia) develop within minutes to several days of the previous criteria; 3) symptoms are clinically significant or impair social, occupational, and other important areas of functioning; and 4) signs and symptoms cannot be attributed to any other medical or mental disorder, including withdrawal from or intoxication with another drug.20
NSAIDs and Acetaminophen
NSAIDs and acetaminophen are often included in the multimodal approach to pain management in opioid-naïve patients because of their opioid-sparing property. Similarly, this approach should be employed in maximally tolerated doses in opioid-tolerant individuals.17 It is suggested that use of these agents will decrease the need and increase the time for rescue medications required for breakthrough pain.7 Both subgroups of medications may be given orally or parenterally.
SPECIAL POPULATIONS AND ACUTE PAIN MANAGEMENT
Geriatrics
Currently, older adults comprise the fastest-growing segment of the world’s population. The number of people worldwide aged 65 years and older was estimated at 508 million in 2008, and by 2040 that number will increase to 1.3 billion.21 With increasing age, the incidence and prevalence of certain pain syndromes also increase. According to the CDC, more than 2.8 million injuries treated annually in emergency departments result from falls.22
Older adults are frequently untreated or undertreated for pain.23 Barriers to effective pain management in older adults include difficulty in adequately assessing pain, underreporting of pain, atypical manifestations of pain, and misconceptions about tolerance and addiction to opioids.23 Additionally, treatment of acute pain in older adults can be challenging given changes in pharmacodynamics and pharmacokinetics.23 Older adults have increased fat mass, decreased muscle mass, and decreased body water, all of which have important implications for drug distribution. A predictable age-related decline in CYP450 function plays a major role in drug metabolism. In addition, polypharmacy is a well-known issue in this population. Multidisciplinary and multimodal approaches to treatment are recommended to optimize treatment response without jeopardizing safety. It is also important to consider the frailty of older adults and the risk of falls.
The medication lists of all older adults should be reviewed comprehensively for drug interactions and CNS-altering agents. In 2015, the Beers Criteria were updated to note that opioids should be avoided if the patient has a history of falls and fractures or is taking three or more CNS-active drugs concomitantly, which increases the risk of falls.24 It is also important to assess renal and/or hepatic function when designing a pain regimen. The maxim “Start low and go slow” should be followed. Perhaps the most important element is to reassess and continue to titrate up or down based on the patient’s response, as well as tolerability.
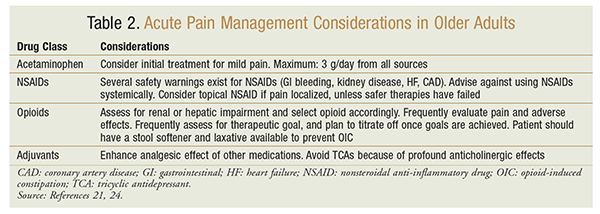
Additionally, an understanding of the different types of pain (nociceptive vs. neuropathic) will guide the use of nonopioids versus adjuvant medications. Adjuvants and topical agents are ideal for geriatric patients to reduce the opioid requirement and associated risks.23 TABLE 2 outlines considerations for acute pain management in older adults.21,24 Using a variety of nonopioid agents can result in favorable outcomes in the treatment of pain in older adults.
Opioid Abuse
Opioid misuse and dependence among prescription-opioid patients continues to rise in the U.S. Opioid addiction is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers.25 Clinicians should be aware of factors associated with opioid abuse that can help predict opioid dependence. Boscarino and colleagues, in a study involving patients receiving long-term opioid therapy, found that opioid dependence is associated with five main factors: age <65 years (odds ratio [OR] 2.33, P = .001), depression (OR 1.29, P = .022), psychotropic medication (OR 1.73, P = .006), history of severe dependence (OR 1.85, P = .001), and previous opioid abuse (OR 3.81, P <.001).26 It is critically important to obtain thorough patient histories in order to be better equipped to detect aberrant behaviors. These risk factors have also been noted to promote perceptions among healthcare providers that can lead to the undertreatment of true pain.
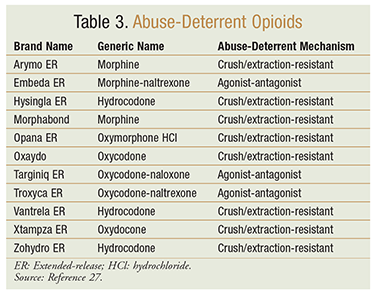
Given the growing epidemic of opioid abuse and misuse, several state boards of pharmacy have implemented prescription-monitoring programs that can help providers identifying aberrant behaviors in the acute-care setting. Additionally, the FDA is encouraging the development of opioid formulations with abuse-deterrent (AD) properties and mixed agonist-antagonist opioids (TABLE 3) to help combat the opioid epidemic.27 Formulations with AD properties target the known or expected routes of abuse, such as crushing in order to snort and dissolving in order to inject. However, most of these newer formulations are extended-release (ER) and are more appropriate for patients requiring long-term opioid use. The FDA notes that long-acting and ER opioid formulations are appropriate only for opioid-tolerant patients.3 ER opioid formulations with AD properties, although usually more expensive than generic opioids, are excellent for managing pain in patients at risk for opioid abuse or misuse.
Long-Term Opioid Agonist Therapy
Increasing numbers of patients with opioid addiction are receiving opioid agonist therapy (OAT) with methadone and buprenorphine, and some are receiving OAT combined with naloxone or simply naltrexone alone. (Naloxone and naltrexone are pure opioid antagonists that block all types of opioid receptors; they can be orally administered and have a long duration of action.) As a result, providers will more frequently encounter OAT patients who have developed painful conditions that need effective treatment strategies. Long-term OAT patients are at increased risk for pain undertreatment.28 NSAIDs and acetaminophen are recommended for treating acute pain; however, if the pain is moderate-to-severe, opioid analgesics may be required for effective control.28
Although it is known that opioid-dependent patients have a reduced pain threshold,28-30 several misconceptions impede effective pain management: 1) maintenance opioids (e.g., methadone) provide analgesia; 2) addiction relapse may occur with the use of opioids for analgesia; 3) respiratory and CNS depression will likely develop with additional opioid use; and 4) reporting of pain may be a manipulation attempt.28 In reality, many patients are reluctant to take opioid analgesics in legitimate situations because of fear of relapse.30 It is suggested that the stress associated with unrelieved pain is more likely than adequate analgesia to trigger relapse.31
This poses a challenge for acute pain treatment but also creates opportunities for education, which is crucially important for both patients and clinicians. A thorough understanding of the mechanisms of action of agents used to treat pain and to manage addiction is paramount. This is particularly true in patients in opioid-substitution programs. For example, patients on extremely high doses of methadone may receive little benefit from additional opioids because opioid receptors are occupied by methadone, and analgesia from methadone does not last long.17 However, splitting the daily methadone dose for administration every 8 hours may be considered to achieve analgesic benefit.17 Another consideration is the use of small doses of methadone as needed in opioid-addicted patients who are currently using illicit opioids to prevent withdrawal symptoms.17
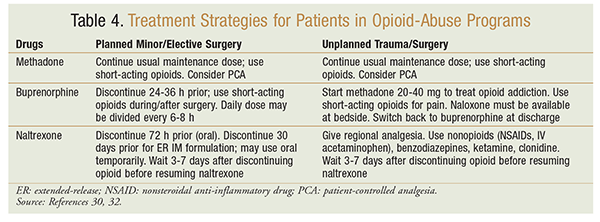
Providers should build a relationship of trust with these patients. The patient’s pain-management plan should be discussed in a nonjudgmental, reassuring manner. The patient should be encouraged to provide a detailed medication history, including prescribed and illicit drugs, in order to promote effective pain management in acute situations. Also, opioid cross-tolerance and increased pain sensitivity, which likely will lead to higher opioid doses required in shorter intervals, should be assessed. Use of a mixed agonist-antagonist opioid for acute pain management should be avoided because these agents can precipitate acute withdrawal symptoms. Maintenance dosing of methadone or buprenorphine should be continued. Any additional analgesia should be provided using a multimodal approach, including nonopioids such as NSAIDs or IV acetaminophen, adjuvant therapies that enhance opioids’ effect, and short-acting opioids.4,32 Adjuvants include ketamine, which has opioid-sparing effects due to N-methyl-d-aspartate receptor antagonism, and alpha2-adrenoceptor stimulators such as clonidine, to reduce opioid-withdrawal symptoms.5,28-30,32 Other treatment strategies for patients in opioid-abuse programs are given in TABLE 4.30,32
CONCLUSION
Acute pain management presents a challenge in patients with opioid tolerance, and undertreatment is a risk in this population. Acute pain should be managed multimodally and with effective coordination of the multidisciplinary team to best meet the patient’s analgesic needs. Patients receiving long-term OAT with methadone or buprenorphine should continue to receive maintenance therapy and may require additional treatment via a multimodal approach, including short-acting opioids, for acute pain management.
REFERENCES
1. Volkow ND. Harnessing the power of science to inform substance abuse and addiction policy and practice. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2015/harnessing-power-science-to-inform-substance-abuse-addiction-policy-practice. Accessed November 29, 2016.
2. Baldwin G. Overview of the public health burden of prescription drug and heroin overdoses. www.fda.gov/downloads/drugs/newsevents/ucm454826.pdf. Accessed November 29, 2016.
3. FDA. Extended-release (ER) and long-acting (LA) opioid analgesics Risk Evaluation and Mitigation Strategy (REMS). www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm311290.pdf. Accessed January 31, 2017.
4. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
5. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39:804-823.
6. Stokowski LA. Adult cancer pain: part 2—the latest guidelines for pain management. Medscape. www.medscape.com/viewarticle/733067. Accessed February 10, 2017.
7. Shah S, Kapoor S, Durkin B. Analgesic management of acute pain in the opioid-tolerant patient. Curr Opin Anaesthesiol. 2015;28:398-402.
8. Prince V. Pain management in patients with substance-use disorders. www.accp.com/docs/bookstore/psap/p7b05.sample03.pdf. Accessed February 10, 2017.
9. Schaible HG, Richter F. Pathophysiology of pain. Langenbecks Arch Surg. 2004;389:237-243.
10. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011;12:996-1004.
11. Bingham B, Ajit SK, Blake DR, Samad TA. The molecular basis of pain and its clinical implications in rheumatology. Nat Clin Pract Rheumatol. 2009;5:28-37.
12. Baratta JL, Schwenk ES, Viscusi ER. Clinical consequences of inadequate pain relief: barriers to optimal pain management. Plast Reconstr Surg. 2014;134(4 suppl 2):S15-S21.
13. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262-268.
14. Katona C, Peveler R, Dowrick C, et al. Pain symptoms in depression: definition and clinical significance. Clin Med (Lond). 2005;5:390-395.
15. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep. 2013;15:421.
16. Wilson PR. Clinical practice guideline: acute pain management. Clin J Pain. 1992;8:187-188.
17. Schug SA. Acute pain management in the opioid-tolerant patient. Pain Manag. 2012;2:581-591.
18. De Andres J, Fabregat-Cid G, Asensio-Samper JM, et al. Management of acute pain in patients on treatment with opioids. Pain Manag. 2015;5:167-173.
19. Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain. 1995;61:195-201.
20. Hasin DS, O’Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834-851.
21. Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J. 2010;10:179-187.
22. Bergen G, Stevens MP, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
23. Cavalieri TA. Management of pain in older adults. J Am Osteopath Assoc. 2005;105(suppl 3):S12-S17.
24. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
25. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
26. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105:1776-1782.
27. FDA. Abuse-deterrent opioids—evaluation and labeling. Guidance for industry. Silver Spring, MD: U.S. Department of Health and Human Services; 2015.
28. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127-134.
29. Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(suppl 4):S3-S13.
30. Vickers AP, Jolly A. Naltrexone and problems in pain management: how to manage acute pain in people taking an opioid antagonist. BMJ. 2006;332:132-133.
31. Karasz A, Zallman L, Berg K, et al. The experience of chronic severe pain in patients undergoing methadone maintenance treatment. J Pain Symptom Manage. 2004;28:517-525.
32. American Society of Addiction Medicine. The ASAM National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






