US Pharm. 2017;42(5)(Specialty&Oncology suppl):22-27.
ABSTRACT: Lung cancer remains the second leading cancer diagnosis and the deadliest form of cancer. Despite recent advances in screening, radiotherapy, immunotherapy, and targeted therapy, overall survival rates continue to be suboptimal. Some of the gene alterations and driver mutations that have been identified include epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS1. Current ALK inhibitors include crizotinib, ceritinib, and alectinib, all of which have demonstrated promising outcomes for patients with ALK-positive lung cancer. ALK inhibitors may cause a variety of adverse events, so pharmacists can assist in creating optimal strategies to manage and prevent adverse events in their patients.
Lung cancer, which is the deadliest form of cancer, will cause an estimated 155,870 deaths (84,590 in males, 71,280 in females) in the United States in 2017. It is projected that approximately 222,500 new cases (116,990 in males, 105,510 in females) will occur in 2017 and that lung cancer will remain the second leading cancer diagnosis, as has been the case for past couple of years.1 Despite recent advances in screening, radiotherapy, immunotherapy, and targeted therapy, the 5-year overall survival rate remains suboptimal (17.7% of all patients with lung cancer).2
Lung cancer can be divided into two major classes: non–small-cell lung cancer (NSCLC) and small-cell lung cancer.3,4 NSCLC accounts for the majority of cases, with adenocarcinoma being the most common type of lung cancer in the U.S.2 Adenocarcinoma subtypes may be associated with gene alterations, most notably epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) gene rearrangements, and ROS1 gene rearrangements. One study that investigated the presence of oncogenic drivers in 1,007 patients with metastatic adenocarcinoma found a frequency of 64%.5 Currently, the National Comprehensive Cancer Network and an international panel recommend testing for EGFR mutations, ALK gene rearrangements, ROS1 rearrangements, and programmed-death-receptor expression in all patients with adenocarcinoma.6 Treatment decisions are initiated based on the histology and results of driver-mutation testing.
ALK Gene Rearrangements
ALK gene rearrangements are found in approximately 2% to 7% of patients with NSCLC.7 ALK gene rearrangements represent a fusion between ALK and various partner genes, such as echinoderm microtubule-associated protein-like 4. Once this fusion occurs, the gene acts as a driver of lung tumorigenesis and oncogenic activity.8 Patients with ALK rearrangements tend to be light smokers or never-smokers (similar to patients with EGFR mutations), male, and younger.8 Although patients with ALK NSCLC have clinical features similar to those in patients with EGFR NSCLC, the two generally are mutually exclusive. Current FDA-approved ALK inhibitors include crizotinib, ceritinib, and alectinib, which are reviewed below and summarized in TABLE 1.9-11
![]()
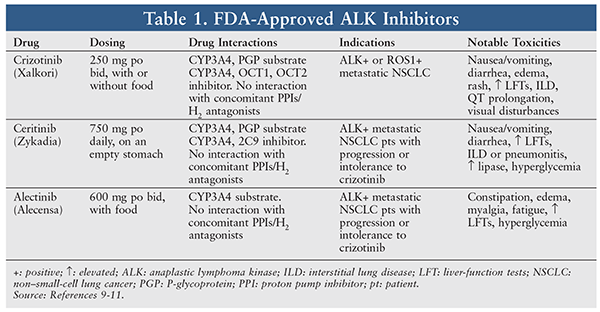
Crizotinib
Crizotinib was the first oral tyrosine kinase ALK inhibitor; it also inhibits hepatocyte growth factor receptor (c-MET), ROS1 (c-Ros), and Recepteur d’Origine Nantais (RON). Crizotinib is currently approved for the treatment of patients with metastatic NSCLC whose tumors are ALK-positive or ROS1-positive.12 The approval and treatment regimens for ALK gene rearrangement were based on a phase II trial (TABLE 2).13,14 Crizotinib has also shown efficacy as a second-line chemotherapy option in the PROFILE 1007 trial (TABLE 2).15
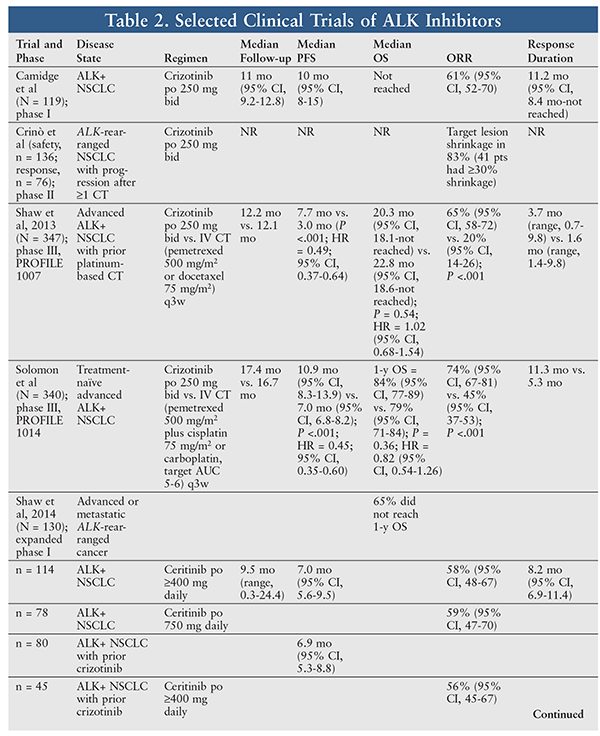
Since its approval, crizotinib has been investigated in a multicenter, randomized, open-label, phase III trial (PROFILE 1014) in adult patients with previously untreated advanced ALK-positive NSCLC. Crizotinib 250 mg twice daily was compared with IV chemotherapy (pemetrexed 500 mg/m2 plus either cisplatin 75 mg/m2 or carboplatin, target AUC of 5-6, every 3 weeks for a maximum of six cycles). Patients were eligible if they had ALK-positive rearrangement; had locally advanced, recurrent, or metastatic nonsquamous NSCLC; and were naïve to systemic treatment. Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, measurable disease, and adequate renal, hepatic, and bone marrow function. Patients with brain metastases that had been treated were also eligible if the metastases were neurologically stable for at least 2 weeks before enrollment and the patient no longer needed glucocorticoids.16
In this trial, 343 patients were randomly assigned in a 1:1 fashion and stratified according to ECOG performance status, Asian race, and presence of brain metastases. The primary end point of median progression-free survival (PFS) was 10.9 months (95% CI, 8.3-13.9) in the crizotinib group and 7.0 months (95% CI, 6.8-8.2) in the chemotherapy group (P <.001; hazard ratio [HR] = 0.45; 95% CI, 0.35-0.60). Overall survival was similar between groups at the time of analysis (P = 0.36; HR = 0.82; 95% CI, 0.54-1.26). The lack of difference could have been due to the fact that 70% of chemotherapy patients crossed over to the crizotinib group, as well as to the overall relatively low rate of death. The objective response rate was 74% (95% CI, 67-81) and 45% (95% CI, 37-53) in the crizotinib and chemotherapy groups, respectively (P <.001). Median duration of response was 11.3 months and 5.3 months, respectively. Notably, 73% of crizotinib patients who had progressive disease continued crizotinib therapy for a median of 3.1 months (range, 0.7-22.6) because of a perceived clinical benefit.16
Compared with the chemotherapy group, the crizotinib group had a statistically significant improvement from baseline in global quality of life in all cycles (P <.001). A clinically meaningful (≥10-point) improvement was found in cycles 9 and 10. Chemotherapy patients also had a diminished global quality-of-life score on days 7 and 15 of cycle 1. From baseline, there was significantly greater worsening of peripheral neuropathy and diarrhea with crizotinib and of nausea and vomiting, appetite loss, fatigue, and alopecia with chemotherapy. Compared with chemotherapy, crizotinib demonstrated significantly greater overall reductions in pain, dyspnea, and insomnia from baseline, as assessed with the quality-of-life core questionnaire (QLQ-C30), and in cough, chest pain, dyspnea, arm or shoulder pain, and pain in other parts of the body, according to the corresponding lung cancer module (Quality of Life Questionnaire Lung Cancer Module [QLQ-LC13]; all, P <.001). Compared with chemotherapy patients, crizotinib patients also had a significantly greater delay in worsening of lung cancer symptoms (P <.001; HR = 0.59; 95% CI, 0.45-0.77).16
Common adverse events (AEs) with crizotinib include vision disorder (71%), diarrhea (61%), edema (49%), vomiting (46%), constipation (43%) and elevated aminotransferases (36%). Most common grade 3/4 AEs included elevated aminotransferase levels (14%) and neutropenia (11%); there were no cases of febrile neutropenia. Overall, 12% of patients permanently discontinued crizotinib due to AEs, most notably elevated aminotransferases and interstitial lung disease (ILD). One case of fatal pneumonitis occurred in a patient who had crossed over to crizotinib.16 Similar toxicity profiles were seen in initial trials of crizotinib.13,14
Ceritinib
Ceritinib is a second-generation oral tyrosine kinase ALK inhibitor that also inhibits insulin-like growth factor-1 receptors, insulin receptors, and ROS1, but not MET.17 In enzymatic assays, ceritinib was shown to be 20 times more potent against ALK than crizotinib.18 It is currently approved for the treatment of patients with metastatic NSCLC whose tumors are ALK-positive and have progressed or are intolerant to crizotinib.10,17
This approval was based on the findings of ASCEND-1, which was an expanded phase I trial of 130 patients who had locally advanced or metastatic ALK cancer (not limited to NSCLC), an ECOG performance status ≤2, and adequate end-organ function. Patients who had received prior treatment with at least one ALK inhibitor and had asymptomatic untreated or treated central nervous system (CNS) metastases also were eligible. Fifty-nine patients were in the dose-escalation phase and 71 were in the expansion phase. Almost all patients (94%) had advanced NSCLC and had received chemotherapy previously; 68% had received crizotinib previously.18
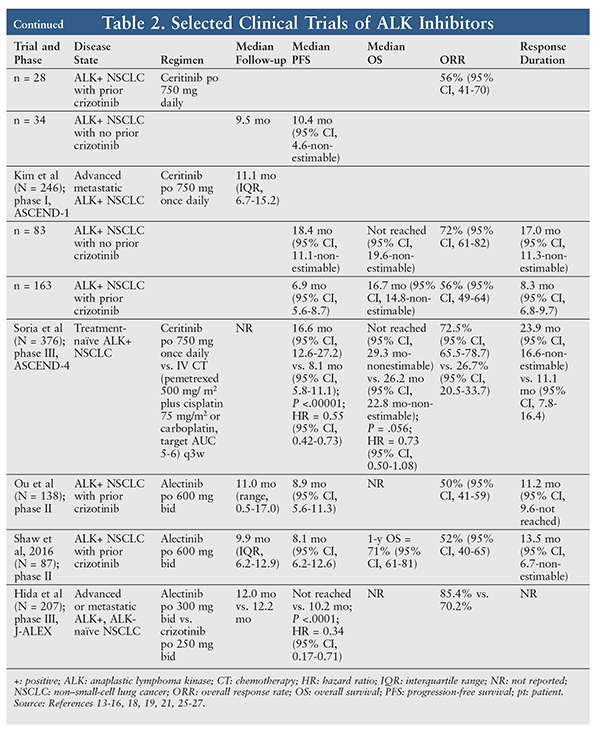
A total of 114 NSCLC patients received ceritinib ≥400 mg daily. One patient (1%) had a confirmed complete response, 65 (57%) had a confirmed partial response, 25 (22%) had stable disease, 12 (11%) had progressive disease, and 11 patients (10%) had an unknown response. The overall response rate (ORR) was 58% (95% CI, 48-67), and 64% (95% CI, 50-74) of responders had a response duration of ≥6 months. Median duration of response in these patients was 8.2 months (95% CI, 6.9-11.4). Median PFS was 7.0 months (95% CI, 5.6-9.5). Of the 78 NSCLC patients who received ceritinib 750 mg daily, 46 had a confirmed partial response. The ORR in these patients was 59% (95% CI, 47-70).18
The ORR for patients previously treated with crizotinib was 56% (95% CI, 45-67) for those receiving ceritinib≥400 mg daily and 56% (95% CI, 41-70) for those receiving ceritinib 750 mg daily. Median PFS was 6.9 months (95% CI, 5.3-8.8) in patients who previously received crizotinib. Of the 34 patients receiving ceritinib ≥400 mg daily who did not receive crizotinib previously, 21 had a partial response and the ORR was 62% (95% CI, 44-78). Median PFS in this subgroup was 10.4 months (95% CI, 4.6-nonestimable). At the time of data cutoff, overall survival data were immature and median overall survival had not been reached. The 1-year overall survival rate was 65%.18
Since this initial report, an updated analysis of ASCEND-1 has been performed. Two hundred and forty-six patients with ALK-positive NSCLC who received at least one dose of ceritinib 750 mg daily were analyzed (83 [34%] ALK inhibitor–naïve and 163 [66%] ALK inhibitor–pretreated). ALK inhibitor–naïve patients achieved an overall response of 72% (95% CI, 61-82), median duration of response of 17.0 months (95% CI, 11.3-nonestimable) and median PFS of 18.4 months (95% CI, 11.1-nonestimable). ALK inhibitor–pretreated patients achieved an overall response of 56% (95% CI, 49-64), median duration of response of 8.3 months (95% CI, 6.8-9.7), and median PFS of 6.9 months (95% CI, 5.6-8.7).19
Ceritinib has shown clinical activity in patients who have received prior ALK-inhibitor therapy and in those who have not. In addition, ceritinib has shown activity with extracranial and intracranial responses in patients with either asymptomatic or controlled brain metastases.18,19 An ongoing confirmatory phase II trial will further investigate ceritinib’s activity in ALK-positive NSCLC with CNS involvement.20
Recently, results of ASCEND-4 were published. ASCEND-4 was a phase III, randomized, open-label study evaluating ceritinib in ALK-positive NSCLC patients who were treatment naïve. Patients receiving ceritinib had a statistically significantly prolonged PFS, a higher response rate, and a longer duration of response compared with patients receiving IV chemotherapy (TABLE 2). This benefit was also seen in patients with brain metastases. Overall survival data in this study are not yet mature.21
The most common grade 1/2 AEs seen with ceritinib in this trial were gastrointestinal—diarrhea (80%), nausea (77%), and vomiting (57%)—and typically occurred within the first month of treatment. Six percent of patients experienced grade 3/4 diarrhea and nausea. The most common grade 3/4 AE was increased alanine aminotransferase (30%), followed by increased aspartate aminotransferase (10%), increased lipase (7%), and hyperglycemia (6%). ILD or pneumonitis was reported in 4% of patients; the majority of cases were severe and included one fatality. Approximately 50% of patients required dose modification, and 11% discontinued treatment because of AEs. Discontinuation of treatment was suspected to be related to the study drug in one-third of cases.19 The safety profile of ceritinib is similar to that of crizotinib. Gastrointestinal AEs occur frequently with both ALK inhibitors, but ceritinib has a higher incidence of severe diarrhea (7% vs. 0%) and nausea (5% vs. 1%). Both drugs can cause elevated liver-function tests.16,19
Alectinib
Like ceritinib, alectinib is a second-generation oral tyrosine kinase ALK inhibitor. It also inhibits RET, but not MET or ROS1. In enzymatic assays, alectinib has been shown to be about five times more potent than crizotinib against ALK. In addition, alectinib can inhibit most of the clinically observed acquired ALK-resistance mutations to crizotinib.22,23 Alectinib is currently approved for the treatment of patients with metastatic NSCLC whose tumors are ALK-positive and have progressed or who are intolerant to crizotinib.11,24 This approval was based on two phase II trials.25,26
One study investigated alectinib use in locally advanced or metastatic ALK-positive NSCLC that had progressed on crizotinib. The 138 patients had a median age of 52 years (range, 22-79 years), 44% were male, two-thirds were white, and 61% had baseline CNS metastasis. The ORR was 50% (95% CI, 41%-59%), the disease-control rate was 79% (95% CI, 70%-86%), and median PFS was 8.9 months (95% CI, 5.6-11.3 months). Similar responses were seen in patients with CNS involvement, including those with a complete CNS response (27%).25 A separate phase II trial investigating alectinib use in ALK-positive NSCLC that had progressed on crizotinib showed similar efficacy (TABLE 2).26
The recent J-ALEX study investigated alectinib head-to-head with crizotinib in ALK inhibitor–naïve, ALK-positive NSCLC patients. Alectinib significantly improved PFS (the primary end point) and ORR after approximately 1-year follow-up. Notably, alectinib reduced the risk of progression by 92% in patients with brain metastases, compared with crizotinib (HR = 0.08; 95% CI, 0.01-0.61). See TABLE 2.27
Overall, alectinib is well tolerated, with 21% of patients requiring a dose reduction or interruption and 8% permanently discontinuing treatment because of an AE.25 The most common AEs were constipation (33%-36%), peripheral edema (23%-25%), myalgia (23%-24%), and fatigue (14%-33%). The incidence of severe AEs that were related to alectinib was low. Death as a result of intestinal perforation and hemorrhage may be linked to alectinib.25,26 Compared with crizotinib, alectinib has a favorable toxicity profile, with lower rates of treatment discontinuation, dose interruptions, and AEs.27
Treatment Strategies, Guidelines, and Future Directions
Currently, crizotinib is recommended as first-line therapy for ALK-positive NSCLC based on its superiority to chemotherapy in the phase III PROFILE 1014 trial.6,16 Crizotinib therapy typically lasts a median of approximately 11 months before the tumor develops resistance and progression occurs.16 When progression takes place, the disease often leads to new intracranial lesions. Ceritinib and alectinib are approved options for patients who have progressed or are intolerant to crizotinib.17,24 Activity of second-generation ALK inhibitors in patients who have progressed on crizotinib suggests that these tumors may remain ALK-dependent and that these agents may overcome some resistant mutations. These second-generation ALK inhibitors have also shown promising results in patients with CNS involvement.18,19,25
Although current guidelines do not recommend ceritinib as a first-line option, results of ASCEND-4 demonstrate that ceritinib is a viable option in patients who have ALK-positive NSCLC and are treatment naïve.21 Ceritinib may also benefit patients who have brain metastases and have not yet undergone treatment. J-ALEX provides evidence that second-generation ALK inhibitors are superior to crizotinib in treatment-naïve ALK-positive NSCLC, especially in patients with brain metastases.27 It is unknown, however, which second-generation ALK inhibitor provides the most benefit. Currently, there are many ongoing or planned phase III first-line trials to compare other newer-generation ALK inhibitors, including brigatinib, ensartinib, and lorlatinib, with crizotinib. In the future, trials comparing newer-generation ALK inhibitors will provide guidance as to which ones have the most activity while maintaining quality of life.
Pharmacist’s Role
Pharmacists can have an impact on the management of patients with ALK-positive NSCLC. They can play a major role in patient adherence to these oral tyrosine kinase inhibitors; further, they can instruct patients on whether to take the medication with or without food to optimize absorption. ALK inhibitors may cause a variety of AEs, so pharmacists can assist in creating optimal strategies to manage and prevent AEs in their patients. Pharmacists should assess patients for drug-drug interactions and monitor laboratory values, as well as recommend any potential dose adjustments, if needed.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Accessed March 28, 2017.
3. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243-1260.
4. Travis WD, Brambilla E, Burke AP, et al. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240-1242.
5. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998-2006.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer. Version 4.2017. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed January 1, 2017.
7. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693-1703.
8. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non–small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247-4253.
9. FDA. Approved drugs: crizotinib. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm376058.htm. Accessed February 7, 2017.
10. FDA. Approved drugs: ceritinib. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm395386.htm. Accessed February 7, 2017.
11. FDA. Approved drugs: alectinib. December 2015. Available from www.fda.gov/drugs/informationondrugs/approveddrugs/ucm476946.htm. Accessed February 7, 2017.
12. Xalkori (crizotinib) package insert. New York, NY: Pfizer Inc; January 2017.
13. Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(suppl 15):abstract 7514.
14. Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol. 2011;29(suppl 15):abstract 2501.
15. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-2394.
16. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167-2177.
17. Zykadia (ceritinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; September 2016.
18. Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med. 2014;370:1189-1197.
19. Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452-463.
20. ClinicalTrials.gov. A phase II study to evaluate the efficacy and safety of oral ceritinib in patients with ALK-positive NSCLC metastatic to the brain and/or to leptomeninges (Ascend-7). https://clinicaltrials.gov/ct2/show/NCT02336451. Accessed March 28, 2017.
21. Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917-929.
22. Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679-690.
23. Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351:215-221.
24. Alecensa (alectinib) package insert. South San Francisco, CA: Genentech USA, Inc; November 2016.
25. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661-668.
26. Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234-242.
27. Hida T, Nokihara H, Kondo M, et al. Randomised phase 3 trial of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lancet [in press].
To comment on this article, contact rdavidson@uspharmacist.com.






