US Pharm. 2018;43(2)(Specialty&Oncology suppl):6-10.
ABSTRACT: Urothelial carcinoma, which is the most common type of bladder cancer, can affect the bladder, ureter, or renal pelvis. Treatment options for this form of cancer include surgery, radiation, and chemotherapy. In recent years, several checkpoint targets have been investigated as alternatives for the treatment of bladder cancer. Recent advances and new treatment options, particularly immunotherapy agents such as avelumab, pembrolizumab, atezolizumab, durvalumab, and nivolumab, have emerged for bladder cancer. Pharmacists can help manage patients’ immunotherapy in a variety of ways.
In 2017, it was estimated that there would be nearly 79,000 new diagnoses of bladder cancer, potentially causing more than 16,000 deaths.1 Urothelial carcinoma, the most common form of bladder cancer, occurs in urothelial cells lining the inside of the bladder. This type of cancer is also known as transitional cell carcinoma because urothelial cells are transitional cells that can change shape and stretch when the bladder is full.2 Urothelial cancers encompass carcinomas of the bladder, renal pelvis, and ureter. They are more common in men than in women and more common in white than in black persons.1
Cigarette smoking is a major cause of urothelial carcinoma. Excessive consumption of analgesics, particularly those containing phenacetin, has been linked to an increased risk of urothelial-tract cancers. Chronic urinary tract inflammation, chronic bladder infections, and upper urinary tract stones have been associated with cancer of the renal pelvis. Occupational exposure to toxins found in processing paints, dyes, metals, and petrolatum increases the risk. Balkan endemic nephropathy, a form of interstitial nephritis, results in progressive inflammation of renal parenchyma and increases the risk of renal pelvis and ureteral cancers. At this time, genetic factors remain undefined.2,3
The most common symptom of urothelial carcinoma is hematuria, which is often painless. Urinary urgency, urinary frequency, and dysuria are seen in bladder and ureteral cancers. Vesical irritation without hematuria is reported in urinary bladder cancer. Advanced disease may be marked by pain, night sweats, fever, weight loss, and anorexia.2,3
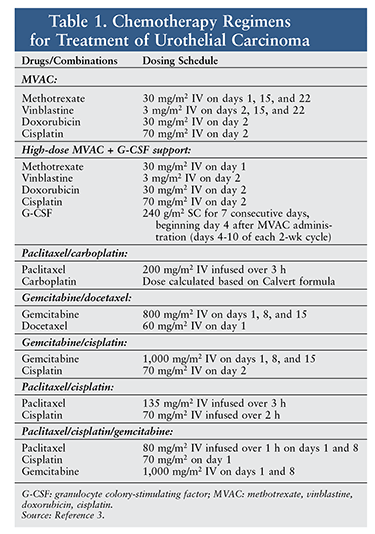
Current pharmacologic treatments for urothelial carcinoma include the combination chemotherapy options listed in TABLE 1. Because of toxicities—such as myelosuppression and mucositis—associated with these therapies and the potential for progression to metastatic disease, newer, less toxic treatment options have been developed. This article will focus on newly approved treatments for urothelial carcinomas.3 Surgery and radiation are beyond the scope of this article.
Pembrolizumab
Pembrolizumab is a programmed death-1 (PD-1) receptor–blocking antibody that recently received accelerated approval to treat patients with locally advanced or metastatic urothelial carcinoma who cannot receive cisplatin-containing chemotherapy. It is also indicated for patients with disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.4,5
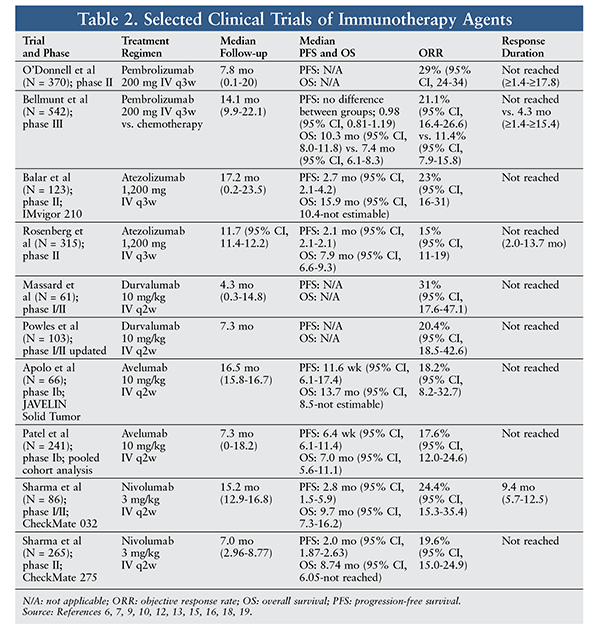
In an ongoing open-label, phase II trial, pembrolizumab 200 mg every 3 weeks was evaluated as first-line therapy in adults with unresectable or metastatic urothelial cancer of the renal pelvis, ureter, bladder, or urethra who are ineligible to receive cisplatin.6 Tumor-response assessments were performed 9 weeks after the first dose, then every 6 weeks for the first year, and every 12 weeks thereafter. The primary endpoint of objective response rate (ORR) was 29% (95% CI, 24-34), and the complete response rate (CRR) was 7%. Median time to response was 2 months (range, 1-5 months), and median duration of response (range, 1-18 months or more) was not yet reached after median follow-up of 8 months (range, 0.1-20 months). Drug-related adverse events (AEs) of any grade occurred in 65% of patients, with 18% being grade 3 or higher.6
An open-label, phase III study evaluated the efficacy of pembrolizumab as second-line therapy for metastatic urothelial carcinoma in 542 patients with advanced urothelial carcinoma that recurred or progressed after initial chemotherapy.7 Patients were randomized to either pembrolizumab 200 mg every 3 weeks or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients receiving pembrolizumab had longer survival and experienced fewer AEs. In the overall population, pembrolizumab patients had a median survival of 10.3 months (95% CI, 8.0-11.8) and chemotherapy patients had a median survival of 7.4 months (95% CI, 6.1-8.3); the hazard ratio (HR) for death was 0.73 (95% CI, 0.59-0.91, P = .002). In the programmed death-ligand 1 (PD-L1)–positive population, median survival was 8.0 months (95% CI, 5.0-12.3) in pembrolizumab patients and 5.2 months (95% CI, 4.0-7.4) in chemotherapy patients; HR for death was 0.57 (95% CI, 0.37-0.88, P = .005). Fewer treatment-related AEs of any grade were reported in the pembrolizumab group compared with the chemotherapy group. The most commonly reported AEs in the pembrolizumab group were pruritus (19.5%), fatigue (13.9%), and nausea (10.9%). Events of grade 3, 4, or 5 were pneumonitis (2.3%), colitis (1.1%), and nephritis (0.8%).7
Atezolizumab
Atezolizumab is a monoclonal antibody immune checkpoint inhibitor that binds to PD-L1. PD-L1 is an immune checkpoint protein expressed on tumor cells and tumor-infiltrating cells that downregulates antitumor t-cell function by binding to PD-1 and the B7 immune-regulatory ligand receptor 1. By blocking PD-L1, atezolizumab restores antitumor t-cell function.8 Atezolizumab is FDA-approved for metastatic and locally advanced or metastatic urothelial carcinoma in patients ineligible for cisplatin-containing chemotherapy, with disease progression during or following platinum-containing chemotherapy, or with disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. 8
In a multicenter, single-arm, phase II trial, Balar and colleagues investigated the safety and efficacy of atezolizumab in metastatic urothelial cancer.9 Of the 123 patients enrolled, 119 received one or more doses of atezolizumab. Patients had no previous treatment for metastatic urothelial cancer, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤2, and a tumor sample for PD-L1 testing. Patients received atezolizumab 1,200 mg every 21 days until occurrence of unacceptable toxicity or investigator-assessed radiographic progression. ORR was 23% (95% CI, 16-31) in all patients after median follow-up of 17.2 months. Complete response was seen in 11 patients (9%), and median time to onset of first response was 2.1 months (range, 1.8-10.5 months). However, not all patients or predefined PD-L1 subgroups reached median response duration. All clinical subgroups assessed had a response to atezolizumab, and 13 of 33 patients (39% [95% CI, 23-58]) with upper-tract primary tumors (renal pelvis or ureter) had an objective response. Median survival in patients older than 80 years was 14.8 months and that in patients with renal dysfunction was 14.1 months, compared with 15.9 months (95% CI, 10.4-not estimable) in all patients.9 Additional trial data are given in TABLE 2.
In this trial, AEs were reported in 114 patients (64%), and 79 patients (66%) had a treatment-related event.9 Treatment-related AEs reported in 10% or more of patients included fatigue, diarrhea, and pruritus. AEs leading to dose interruption were reported in 41 patients (43%), with no single AE predominating. Immune-mediated all-grade AEs were reported in 14 patients (12%). Thirty-six patients received corticosteroids, and no patients received systemic noncorticosteroid immunomodulatory drugs. In the 102 patients (86%) who discontinued treatment, the cause was disease progression in most cases (n = 77).9
In another multicenter, single-arm, phase II trial, the safety and efficacy of atezolizumab for metastatic urothelial cancer were investigated in 315 patients, 311 of whom received atezolizumab 1,200 mg.10 Patients were treatment naïve and ineligible for cisplatin treatment or had progressed after previous platinum-based chemotherapy. Patients were required to have an ECOG PS <2, as well as adequate hematologic and organ function.10 ORR was 15% (95% CI, 11-19), and CRR was 5% in 15 of 310 patients; median time to first response was 2.1 months (95% CI, 2.0-2.2).10 Owing to pseudoprogression, 121 patients were treated beyond progression for a median of 7.8 weeks, with 20 (17%) patients experiencing target-lesion reduction of at least 30% from baseline. Patients were treated for a median of 12 weeks (range, 0-66 weeks). Durable responses were seen in patients with liver metastases, although they were objectively lower compared with those in patients without liver metastases. Median duration of response was not reached at the time of data cutoff.10 Treatment-related AEs occurred in 215 patients (69%), with 50 patients (16%) experiencing grade 3/4 treatment-related AEs. No treatment-related deaths occurred during the study. Most AEs were mild-to-moderate, including fatigue (30%), nausea (14%), decreased appetite (12%), pruritus (10%), pyrexia (9%), diarrhea (8%), and arthralgia (7%). The most common grade 3/4 AE was fatigue, which occurred in five patients (2%). No cases of febrile neutropenia were reported and no immune-mediated renal toxicity was observed. Eleven patients (4%) had an AE that led to treatment withdrawal, and 69 (22%) patients had an AE requiring use of systemic corticosteroids.10
Durvalumab
Durvalumab is FDA-approved for the treatment of patients with locally advanced or metastatic urothelial cancer who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.11
Durvalumab was evaluated in a multicenter, open-label, phase I/II study involving 61 patients who received durvalumab 10 mg/kg every 2 weeks for up to 12 months.12 Patients had confirmed inoperable or metastatic transitional-cell carcinoma and had progressed on, been ineligible for, or refused previous therapies, and they had an ECOG PS <2. At the data cutoff, 42 patients were evaluated for response, and all patients were included in the safety analysis.12 Most patients (93.4%) had received at least one prior systemic therapy, and the population’s median age was 66 years. ORR was 31.0% (95% CI, 17.6 to 47.1) overall, 46.4% in the PD-L1–positive subgroup, and 0% in the PD-L1–negative subgroup. Median time to response was 6.3 weeks (95% CI, 5.6-12.1 weeks) in the 13 responding patients, and all but one had an ongoing response at last follow-up.12
In a recent study of durvalumab, 103 patients were included in the primary efficacy analysis.13 Of those, 21 patients (20.4%, 95% CI,13.1-29.5) had a confirmed response and 5 patients (4.9%) had a complete response. Responses were seen in both PD-L1–high and PD-L1–low/negative subgroups. Durable response was seen at a median of 1.4 months, and 18 patients had an ongoing response.13 Grade 3/4 treatment-related AEs were low: Grade 3/4 immune-mediated AEs occurred in three patients (5.2%), resulting in one discontinuation secondary to acute kidney injury. A dosage of 10 mg/kg every 2 weeks had clinical activity and a good safety profile in patients with urothelial cancer.13
Avelumab
Avelumab is FDA-approved for the treatment of patients with locally advanced or metastatic urothelial cancer who have disease progression during or following platinum-containing chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.14
JAVELIN Solid Tumor is an ongoing open-label, phase I trial investigating the tolerability, safety, and clinical activity of avelumab in patients with metastatic or locally advanced solid tumors with expansion in selected tumor types.15 The phase Ib dose-expansion cohort included patients with metastatic urothelial cancer of the renal pelvis, ureter, urinary bladder, or urethra. Eligible patients received avelumab 10 mg/kg over a 1-hour infusion every 2 weeks until predefined criteria for withdrawal occurred.15 All patients receiving avelumab experienced an AE, and 29 of 44 (65.9%) experienced a treatment-related AE. The most common treatment-related AEs were fatigue (20.5%), infusion-related reaction (20.5%), asthenia (11.4%), and nausea (11.4%). Three patients experienced grade 3/4 treatment-related AEs (elevated blood creatinine phosphokinase and aspartate aminotransferase, asthenia, and decreased appetite).15 At the time of analysis, 13 patients (29.5%) had a reduction in target lesion of 30% or more from baseline. In patients evaluable for PD-L1 expression, responses occurred in those who were PD-L1–positive (75.5% [95% CI, 41.6-91.4]) and those who were PD-L1–negative (56.3% [95% CI, 33.7-73.9]) at all prespecified PD-L1 expression thresholds.15
Patel and colleagues analyzed two pool cohorts from the JAVELIN Solid Tumor trial. Patients were included if they had metastatic urothelial carcinoma and progressed after platinum-based therapy or were cisplatin-ineligible and unselected for PD-L1 expression.16 Of these patients, 95.4% had disease progression on previous platinum therapy. Patients received avelumab 10 mg/kg every 2 weeks. At 24 weeks, the response duration was 92% (95% CI, 71.6-97.9), with 36 patients having stable disease as best response. ORRs for PD-L1–positive patients and PD-L1–negative patients were, respectively, 25% (95% CI, 14.4-28.4) and 14.7% (95% CI, 7.6-24.7). Treatment-related AEs of any grade that occurred in 10% of patients were infusion-related reaction (22.8%) and fatigue (12%). Immune-related and treatment-related AEs of any grade occurred in 28 patients (11.6%), with 2.5% of patients having a grade 3 or higher event. There was one treatment-related death caused by pneumonitis.16
Nivolumab
Nivolumab is FDA-approved for the treatment of patients with locally advanced or metastatic urothelial cancer who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.17
An analysis of the ongoing CheckMate 032 trial included patients diagnosed with urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra with an ECOG score <2 and measurable disease by CT scan or MRI.18 Patients had disease progression after at least one previous platinum-based chemotherapy treatment for metastatic or locally advanced unresectable disease, with recurrence within 1 year of completing previous platinum-based treatment, or had refused standard chemotherapy for metastatic disease. Patients received nivolumab 3 mg/kg every 2 weeks until disease progression, treatment discontinuation due to unacceptable toxicity, or other protocol-defined reasons. In response-evaluable patients, change in tumor burden over time occurred in 31 patients (40%) who continued nivolumab monotherapy beyond progression. Of these patients, nine had nonconventional benefit (i.e., did not have a best overall response before progression and met at least one of the predefined criteria, including appearance of a new lesion).18 Grade 3/4 treatment-related AEs occurred in 17 patients (22%), most commonly elevated lipase (5%); elevated amylase (4%); and fatigue, maculopapular rash, dyspnea, decreased lymphocyte count, and decreased neutrophils (each, 3%). Serious AEs, including colitis, diarrhea, mouth ulceration, nausea, thrombocytopenia, hyponatremia, acute kidney injury, and pneumonitis, were reported in 36 patients (46%). Two patients (3%) discontinued treatment because of grade 4 AEs (pneumonitis and thrombocytopenia). In this study, nivolumab elicited a substantial and durable tumor response in patients with locally advanced or metastatic urothelial carcinoma.18
In the phase II CheckMate 275 trial, patients received nivolumab 3 mg/kg every 2 weeks until disease progression, clinical deterioration, unacceptable toxicity, or another protocol-defined reason.19 At the time of analysis, 66 patients (24%) were still on the study treatment. The most common reason for discontinuation was disease progression, which occurred in 144 patients (53%). Seventy patients (26%) were treated with nivolumab beyond progression, and of those, 24 (34%) were deemed to have experienced nonconventional benefits. Nivolumab demonstrated activity across all predefined subgroups, which included baseline site of metastasis, ECOG performance score, tumor origin, and previous chemotherapy regimen used. Treatment-related AEs occurred in 174 patients (64%); the most common AE was fatigue. Grade 3/4 treatment-related AEs occurred in 48 patients (18%), including fatigue and diarrhea. The most common immune-mediated, treatment-related AEs were skin and endocrine AEs, which were manageable with systemic corticosteroids. In the safety-population analysis, 138 deaths (51%) were reported, of which 121 (88%) were due to disease progression. In conclusion, nivolumab monotherapy showed clinical benefit irrespective of PD-L1 expression and showed an acceptable safety profile in patients previously treated for metastatic or surgically unresectable urothelial carcinoma.19
Although nivolumab was studied as a 3 mg/kg every-2-week dosing schedule, its current approved dosing for locally advanced or metastatic urothelial cancer is a flat 240 mg every 2 weeks. This was based on pharmacokinetic analyses and dose/exposure-response analyses demonstrating comparability of the pharmacokinetics exposure, safety, and efficacy of the flat-dosing regimen with the weight-based regimen.20
Treatment Strategies, Guidelines, and Future Directions
Immunotherapy in patients with locally advanced or metastatic bladder cancer who are ineligible for platinum-based chemotherapy or have disease progression after traditional chemotherapy has evolved tremendously over the past few years. This has offered hope to many patients who previously had few options. Several agents, such as durvalumab and pembrolizumab, have accelerated approval based on tumor-response rates, and continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials. The improved safety and tolerability of these agents versus cisplatin and other chemotherapy drugs will allow patients to receive treatment for a longer time.
Atezolizumab and pembrolizumab are approved as first-line options in patients who are unable to tolerate cisplatin-containing chemotherapy for locally advanced or metastatic urothelial cancer. The National Comprehensive Cancer Network recommends pembrolizumab as a category 1 therapy; atezolizumab, nivolumab, durvalumab, and avelumab are second-line systemic therapy options following platinum-based therapy (category 2A).21 Platinum-based chemotherapy, which was the standard of care in metastatic disease prior to immunotherapy, gave patients an overall survival of 9 to 15 months.22,23 To date, all immunotherapy agents also have shown durable responses in patients with bladder cancer and could surpass the overall survival of traditional chemotherapy.
Pharmacist’s Role
Pharmacists can play an important role in helping oncologists manage patients’ immunotherapy by ensuring that patients receive the correct doses; assessing for potential drug-drug interactions and drug-disease interactions; monitoring laboratory values; and minimizing side effects. Pharmacists can optimize premedications in order to improve patient tolerability. They can also follow up with patients to identify potential treatment-related AEs and to provide supportive-care recommendations and strategies to prevent future AEs.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. National Cancer Institute. Bladder cancer treatment (PDQ®)—health professional version. www.cancer.gov/types/bladder/hp/bladder-treatment-pdq. Accessed August 25, 2017.
3. Hurwitz M, Spiess PE, Garcia JA, Pisters LL. Urothelial and kidney cancers. www.cancernetwork.com/cancer-management/urothelial-and-kidney-cancers. Accessed August 25, 2017.
4. Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck & Co, Inc; September 2017.
5. FDA. Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm559300.htm. Accessed August 21, 2017.
6. O’Donnell PH, Grivas P, Balar AV, et al. Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC) [abstract 4502]. J Clin Oncol. 2017;35(suppl).
7. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015-1026.
8. Tecentriq (atezolizumab) package insert. South San Francisco, CA: Genentech, Inc; April 2017.
9. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67-76.
10. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-1920.
11. Imfinzi (durvalumab) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; April 2017.
12. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119-3125.
13. Powles T, O’Donnell PH, Massard C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma [abstract 286]. J Clin Oncol. 2017;35(suppl 6S).
14. Bavencio (avelumab) package insert. Rockland, MA: EMD Serono, Inc; March 2017.
15. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35:2117-2124.
16. Patel MR, Ellerton JA, Infante JR, et al. Avelumab in patients with metastatic urothelial carcinoma: pooled results from two cohorts of the phase 1b JAVELIN Solid Tumor Trial [abstract 330]. J Clin Oncol. 2017;35(suppl 6S).
17. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; September 2017.
18. Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590-1598.
19. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312-322.
20. FDA. Modification of the dosage regimen for nivolumab. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm520871.htm. Accessed August 21, 2017.
21. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: bladder cancer. Version 5.2017. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed August 21, 2017.
22. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602-4608.
23. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II—results of EORTC study 30986. J Clin Oncol. 2009;27:5634-5639.
To comment on this article, contact rdavidson@uspharmacist.com.






