ABSTRACT: Clostridium difficile infection (CDI) is a significant public health concern with increasing prevalence over the past decade; considerable morbidity and mortality is associated with the infection. Pharmacists play an important role in both the prevention and treatment of CDI. Metronidazole, oral vancomycin, and fidaxomicin are antibiotic treatment options. A monoclonal antibody has entered the market for recurrence prevention, and vaccines are in development for primary prevention.
US Pharm. 2017;42(12):HS-7-HS-12.
Clostridium difficile infection (CDI) is the most common healthcare-associated infection.1-3 In 2010, 55.7% of hospitalized patients received antibiotics; it is estimated that a 30% reduction in broad-spectrum antibiotic use would decrease CDI by 26%.4 Annual incidence includes 453,000 cases, with 83,000 recurrence episodes and 29,300 deaths within 30 days of diagnosis.2,5 Over 80% of deaths from CDI occur in patients aged 65 years and older.5 The CDC has listed CDI as an urgent threat.6 Approximately 65.8% of CDI is healthcare-associated; however, patients experience symptom onset before discharge in only 24.2% of cases. Recurrence and reinfection rates range from 20% to 40%.1,2 CDI is the most common cause of antibiotic-associated diarrhea, causing 15% to 39% of cases, and is responsible for more than $4.8 billion in additional healthcare costs each year in acute-care facilities.2,7 CDI rates doubled from 2000 to 2003, but progress is being made in reducing incidence.7 The 2013 National and State Healthcare-Associated Infections Progress Report found a 10% decrease in CDI between 2011 and 2013.5
Etiology
C difficile is a gram-positive, spore-forming, anaerobic, toxin-producing bacillus that is transmitted via the fecal-oral route.8,9 Spores are typically spread via unclean surfaces, through contact with contaminated feces and/or dirty hands, and through failure to notify the receiving healthcare facility when patients with an existing CDI infection are transferred from another healthcare facility.5 The bacterial spore is the etiologic agent of CDI.7 These spores are ubiquitous, serving as a continuous source for infection through their tenacious adherence to fomites (i.e., bedding).7 The spores are resistant to environmental insults (e.g., pH and temperature), and transmission typically occurs from workers and the environment to patients.7
Diarrhea and colitis are a result of the release of two homologous glycosylating endotoxins by pathogenic C difficile strains, toxin A, an enterotoxin, and toxin B, a cytoxin.8-10 Toxin A (TcdA) is responsible for increased intestinal permeability and fluid secretion, while toxin B (TcdB) results in intense colonic inflammation. These toxins bind to receptors to gain intracellular entry when healthy bacterial flora has been disrupted, allowing growth and colonization. Binding results in a loss of intracellular tight junctions, leading to destruction of mucosal tissue by inflammatory responses.9 During the past decade, highly virulent epidemic strains (i.e., BI/NAPI/027) have emerged, with greater morbidity and mortality. A third virulence factor is produced by these hypervirulent strains, binary toxin (CDT), whose role in pathogenesis is not well understood.9
Approximately 30% of patients admitted to hospitals in the United States are asymptomatic carriers of C difficile, with prevalence increasing to 50% in patients with a history of long-term hospitalization. Although presence of asymptomatic carriers in nosocomial settings increases the risk of transmission to others, it does not increase the risk of CDI in the carrier and may actually be protective against symptomatic disease. There is no indication for treatment in asymptomatic carriers.8 Approximately 60% of healthy adults are thought to have detectable serum IgG and IgA antibodies to C difficile toxins, even in the absence of colonic/active infection.10
Risk Factors
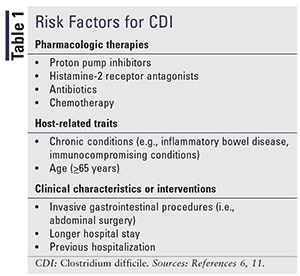
Risk factors for CDI are summarized in TABLE 1. There have been more than 40 risk factors associated with CDI or recurrent CDI.11 It is challenging to identify the full impact of many of the theorized risk factors, owing to issues of high heterogeneity, bias, and confounding in the available literature. A large, prospective study assessing risk factors for CDI with standardized case definitions and stratification is necessary to develop a more accurate and robust estimate of risk factors and their impact on CDI and recurrent CDI.11
Additional pharmacological therapies have been implicated in CDI, but evidence is limited or mixed compared with the therapies listed in TABLE 1. There is emerging evidence that nonselective nonsteroidal anti-inflammatory drugs and corticosteroids may be associated with increased prevalence. Antibiotic therapy is the most relevant risk factor, increasing likelihood of CDI 6 to 10 times.5,11 More than half of hospitalized patients receive antibiotics, but 30% to 50% are inappropriate or unnecessary.5 Clindamycin, broad-spectrum cephalosporins, and fluoroquinolones produce the greatest risk, but carbapenems and trimethoprim/sulfamethoxazole are also associated. Aminoglycosides and macrolides have not been found to increase the risk of healthcare-associated CDI. Continued use of a causative antibiotic during follow-up has also been found to increase the risk of recurrent CDI.11
Proton pump inhibitors (PPIs) may have a significant impact on CDI; according to one study, 36% of patients diagnosed with CDI did not receive an antibiotic 12 weeks before diagnosis, but 31% of those patients were taking a PPI.12 Host-related traits that can increase CDI risk include immune-suppressing conditions or medications (e.g., rheumatoid arthritis, kidney transplant, cancer), and gastrointestinal disorders; other areas are emerging, such as diabetes.7 Renal disease has been associated with a greater recurrence, likely related to immune dysfunction, frequent antibiotic use, and frequent contact with the healthcare system. Risk is greater for patients with chronic kidney disease who are on dialysis compared with those who are not.1 Extended care at a healthcare facility increases risk by increasing the likelihood of exposure to spores by two to four times.7,11 A study of four surgical centers found a greater likelihood of CDI in patients undergoing surgery who had antibiotic abuse, an increased American Society of Anesthesiologists score (a classification scale widely used for stratification of preoperative health of surgical patients), and increased hospital admissions in the preceding year.13
Symptoms and Diagnosis
The presence of symptoms associated with CDI varies significantly, ranging from carriers who are asymptomatic to patients with fulminant colitis and multiorgan failure. Disease severity classifications are presented in TABLE 2. CDI typically presents as watery diarrhea with a foul odor occurring every 1 to 2 hours with abdominal cramps, subfebrile temperature, and leukocytosis. Leukocytosis can precede diarrhea by 1 to 2 days. Physical exams usually produce lower-abdominal tenderness. A clinically significant fever is a sign of more severe disease. If CDI occurs as a result of antibiotic exposure, symptoms usually present within 2 weeks of antibiotic use.8
There are two treatment guidelines available: one from the Society for Healthcare Epidemiology of America (SHEA) with the Infectious Diseases Society of America (IDSA), and a separate statement by the American College of Gastroenterology (ACG).14,15 The SHEA/IDSA guidelines were released in 2010, with an update currently in progress; the ACG guidelines were released in 2013.14,15 Two criteria are necessary for diagnosis: 1) presence of diarrhea with three or more loose, watery stools in a 24-hour period and 2) a positive stool test identifying the presence of toxigenic C difficile or its toxins, or identification of pseudomembranous colitis through colonoscopic/histologic pathology findings.5,8,14 Only loose stools should be tested, as there is frequently short-term asymptomatic excretion of C difficile in hospitals as a result of close proximity, recent antibiotic exposure, and free movement of spores.9,16 Testing for a cure and repeat testing are both discouraged.9
Several tests confirm CDI; each has pros and cons. Real-time polymerase chain reaction (RT-PCR) and nucleic amplification tests (NAAT) are the standard of care.9 One limitation of RT-PCR testing is that it detects toxin genes but does not indicate if these are active toxins, and it is not quantitative, so the value in asymptomatic carriers is unclear. RT-PCR testing is also potentially limited by the prevalence of asymptomatic carriers in the population being treated. The fecal enzyme immunoassay (EIA) is also commonly used because results are returned rapidly, but it lacks sensitivity. The EIA can be utilized in a two-step confirmatory process, using EIA to test for glutamate dehydrogenase and confirming by NAAT.9 The most sensitive test available is the cell culture cytotoxicity assay, but it is not used frequently because of its long return time.16 Endoscopy is required to diagnose pseudomembranous colitis, which occurs in up to 50% of CDI cases.16
Emerging Therapies
Research surrounding new therapies has been a focus in the last decade because of the increasing prevalence of CDI and resistant and pathogenic strains. Therapies investigated include vaccines, monoclonal antibodies, new antibiotics, and synthetic stool products. Several vaccines are currently in development that contain modified C difficile toxins to lead a serum antibody response, resulting in seroconversion.6 Study vaccines include chimeric fusion proteins of TcdA, TcdB, and CDT in both trivalent (T-toxin, CDTb/TcdB(003)/TcdA) and quadravalent (Q-toxin, CDTb/TcB(003)/TcdA/TcdB(027)) vaccines.3 Another vaccine, a DNA vaccine that targets TcdA and TcdB, is in earlier stages of development; it shows neutralizing activity both in vitro and in vivo.17
Previously, most vaccines provided immunity against a single antigen, but with antibiotic-resistant genes being discovered on the C difficile genome, vaccines must now target more antigens for a chance at efficacy.17 The vaccines furthest along in development are all three-dose series that would be indicated for primary prevention. Considerable efforts by pharmacists and other members of the healthcare team would be required, therefore, to identify patients at greatest risk for CDI.6 The vaccines would not be indicated for prevention of recurrent infections.
One vaccine, Cdiffense (NCT01887912), is an intramuscular toxoid vaccine that contains formalin-inactivated TcdA and TcdB, manufactured by Sanofi. This vaccine is currently in phase III trials and is being evaluated for prevention up to 3 years following vaccination. Another vaccine, VLA84, made by Valneva Austria, is a recombinant fusion protein vaccine of TcdA and TcdB binding regions (NCT02316470). It is currently in phase II trials to confirm safety and identify the optimal dose and formulation. Lastly, Pfizer has a phase III trial (NCT02561195) of a genetically modified and chemically treated recombinant, full-length TcdA and TcdB vaccine. If approved, these vaccines would initially be indicated for patients aged more than 50 years for primary prevention of CDI.6
Bezlotoxumab (Zinplava) was approved in 2016 following the MODIFY I and MODIFY II trials for prevention of recurrent CDI.6 Bezlotoxumab is a fully human monoclonal antibody and provides passive immunity via binding and neutralizing TcdA and TcdB. In these phase III trials, bezlotoxumab and actoxumab were compared with placebo and the standard-of-care antibiotic for prevention of recurrent CDI. Sustained cure (cure at 12 weeks) was found to be 64% versus 54% for placebo, while actoxumab was found to have lower efficacy and serious side effects, and has not been brought to market.18 Although bezlotoxumab does not decrease inpatient stay time, it does decrease recurrent CDI compared with placebo, particularly in elderly patients and patients with epidemic BI/NAPI/027. Recurrence was found to be 8% with monoclonal antibody compared with 32% with placebo in patients having the B1/NAP1/027 strain. Patients older than age 65 years had a 51% lower recurrence of CDI compared with placebo.18,19
Innovative fecal-transplantation delivery approaches are being investigated. The ideal route of administration is unknown; therefore, innovative dosage forms (e.g., encapsulated stool, frozen preparations) are being tested. Frozen preparations would remedy issues of availability and suitability of donors. Synthetic and purified stools are being researched, as human stool is constantly changing and bacterial species that are thought to establish healthy fecal flora can be offered without the challenges of current fecal samples (e.g., cytomegalovirus, antibiotics in previous 3 months, history of gastrointestinal disorder).20
Treatment
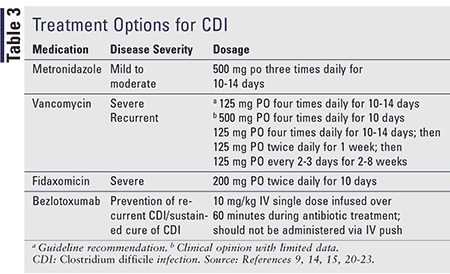
Treatment for CDI, as described in TABLE 3, has remained relatively consistent despite new therapies, owing to a absence of guideline updates since these therapies entered the market. The SHEA/IDSA update should provide greater clarification of the role of bezlotoxumab, and fidaxomicin, discussed below. Contact precautions (e.g., gown, gloves, isolation rooms) with hospitalized patients who have CDI remain a foundation of prevention.6
Prior to antimicrobial treatment, CDI-causing medications should be stopped immediately.9
Metronidazole: Metronidazole is first-line therapy in mild-to-moderate CDI unless the patient has an allergy or intolerance, is pregnant, or is breastfeeding. Patients who meet these criteria should be treated with oral vancomycin.15
Vancomycin: Vancomycin is usually reserved for severe cases, cases of intolerance or contraindicatations to metronidazole, and for those who do not respond to metronidazole. Vancomycin and metronidazole have comparable cure (84%-95%; 74%-94%) and relapse rates (7%-17%; 5%-21%). Vancomycin and metronidazole are equally effective in treating mild-to-moderate disease, whereas vancomycin is superior in severe disease.21 Oral vancomycin is preferred in pregnant and nursing patients. Patients who do not respond to metronidazole within 5 to 7 days should be switched to vancomycin.9 Vancomycin may offer a shorter time to diarrhea resolution. Metronidazole should not be used ≥14 days and after first recurrence because of hepatoxicity and polyneuropathy risk.21
Fidaxomicin: Fidaxomicin and vancomycin have similar cure rates; however, sustained cure rates are significantly higher in fidaxomicin, with lower recurrence rates. Fidaxomicin is better than vancomycin for prevention of a second recurrence.9 Despite clinical benefit, fidaxomicin’s use has been limited by cost and, potentially, by lack of preferential recommendation in guidelines. Fidaxomicin provides higher global cure rates and lower recurrence in patients with non-B1/NAP1/027 strains and allows for more rapid restoration of and minimal effect on normal microflora because it lacks gram-negative bacterial activity.20,22 Fidaxomicin is ideal for patients with severe disease or risk factors for recurrence.22
A first relapse should be treated with the same treatment as the initial course, while a second relapse should be treated with oral vancomycin taper, intermittent oral vancomycin, or fidaxomicin. A third relapse would warrant consideration of fecal microbiota transplant with vancomycin.9 By using oral vancomycin tapers, there is time for restoration of the normal flora.21
Conclusion
CDI remains a prevalent public health concern in the U.S. Emerging therapies may play a role in both primary prevention of CDI and limiting recurrence in patients who have had CDI previously. Facilitating appropriate antibiotic and PPI use are areas where pharmacists can impact this public health issue.
REFERENCES
1. Safdar N. Clostridium difficile: the emerging epidemic. Mayo Clin Proc. 2012;87(11):1037-1039.
2. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. New Engl J Med. 2015;372(9):825-834.
3. Tian JH, Glenn G, Flyer D, et al. Clostridium difficile chimeric toxin receptor binding domain vaccine induced protection against different strains in active and passive challenge models. Vaccine. 2017;35(33):4079-4087.
4. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. Morb Mortal Wkly Rep. 2014;63(9):194-199.
5. CDC. Healthcare-associated Infections: Clostridium difficile infection. www.cdc.gov/hai/organisms/cdiff/cdiff_infect.html. Accessed September 1, 2017.
6. Legenza LM, Barnett SG, Rose WE. Vaccines in development for the primary prevention of Clostridium difficile infection. J Am Pharm Assoc. 2017;57(4):547-549.
7. Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: an overview of the disease and its pathogenesis, epidemiology, and interventions. Gut Microbes. 2010;1(4):234-242.
8. Postma N, Kiers D, Rickkers P. The challenge of Clostridium difficile infection: overview of clinical manifestations, diagnostic tools and therapeutic options. Int J Antimicrob Agents. 2015;46(suppl 1):S47-S50.
9. Ofosu A. Clostridium difficile infection: a review of current and emerging therapies. Ann Gastroenterol. 2016;29(2):147-154.
10. Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070-1079.
11. Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections—an overview of the evidence base and challenges in data synthesis. J Glob Health. 2017;7(1):010417.
12. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Internal Med. 2013;173(14):1359-1367.
13. Bernatz JT, Safdar N, Hetzel S, Anderson PA. Antibiotic overuse is a major risk factor for Clostridium difficile infection in surgical patients. Infect Control Hosp Epidemiol. 2017;38(10):1254-1257.
14. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
15. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
16. Dupont HL. Diagnosis and management of Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1216-1223.
17. Zhang BZ, Cai J, Yu B, et al. A DNA vaccine targeting TcdA and TcdB induces protective immunity against Clostridium difficile. BMC Infect Dis. 2016;16(1):596.
18. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317.
19. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3):197-205.
20. Cornely OA. Current and emerging management options for Clostridium difficile infection: what is the role of fidaxomicin? Clin Microbiol Infect. 2012;18 (suppl 6):28-35.
21. Tart SB. The role of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea. J Pharm Pract. 2013;26(5):488-490.
22. Juang P, Hardesty JS. Role of fidaxomicin for the treatment of clostridium difficile infection. J Pharm Pract. 2013;26(5):491-497.
23. Varughese CA, Vakil NH, Phillips KM. Antibiotic-associated diarrhea: a refresher on causes and possible prevention with probiotics—continuing education article. J Pharm Pract. 2013;26(5):476-482.