US Pharm. 2018:6(43)(Generics suppl):16-22.
ABSTRACT: Over the last decade generic drugs have saved the United States healthcare system well over a trillion dollars. Patients abandon generic drugs at a lower rate than brand-name drugs. Recognizing that getting generic-drug alternatives to the market is a public health concern, the FDA approved a record number of Abbreviated New Drug Applications in 2017, including several significant first generics. In 2018, anticipated first generics include agents for central nervous system, dermatologic, and urologic disease and anti-inflammatory and antiretroviral agents, among others.
Over the last decade, generic drugs have saved the United States healthcare system $1.67 trillion dollars, and in 2016 saved $253 billion.1 Furthermore, according to third-party researchers, patient abandonment rates for brand-name products were 266% higher when compared with generic drugs.1 Given the impact generic drugs have on patient care, the FDA has implemented processes to improve the efficiency of generic-drug approvals, leading to a record number of first-generic approvals.2
According to the 2017 annual report of the Office of Generic Drugs, the FDA approved 843 Abbreviated New Drug Applications (ANDAs) and tentatively approved 184 ANDAs, for a total of 1,027 approvals—a record number.2 The FDA attributes the increase in application approvals and efficiency to the implementation of the Generic Drug User Fee Amendments of 2012, in which user fees are paid by the pharmaceutical industry. These fees are thought to have allowed for the additional resources needed to review and approve applications in a more timely and predictable process. In 2017, Congress reauthorized the program for an additional 5 years. The FDA recognizes that getting generic-drug alternatives to the market is a public health concern.2
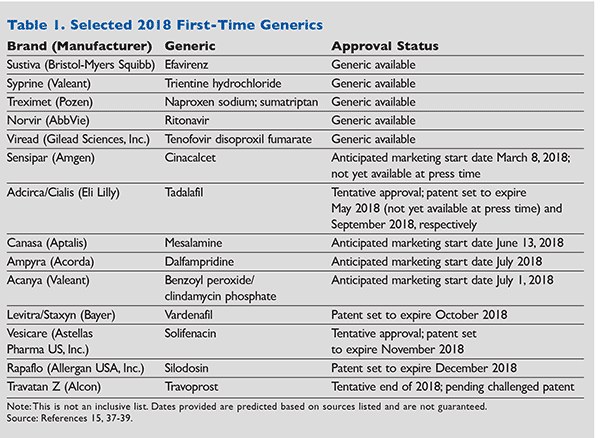
Included in the ANDAs approved in 2017 were some significant first-generic approvals: Strattera (atomoxetine), indicated for attention deficit/hyperactivity disorder; Truvada (emtricitabine/tenofovir), indicated for HIV treatment; Vytorin (ezetimibe/simvastatin), indicated for hyperlipidemia; Asacol (mesalamine DR), indicated for treatment of ulcerative colitis; Renvela (sevelamer), indicated for treatment of hyperphosphatemia; and Effient (prasugrel), an antiplatelet.2 This article will briefly discuss selected first generics that are anticipated in 2018, listed in Table 1.
Central Nervous System Agents
Treximet (sumatriptan and naproxen sodium): The newly approved, AB-rated generic equivalent of Treximet, sumatriptan and naproxen sodium, was released in February 2018. This combination generic is FDA-approved for the treatment of acute migraine with or without aura.3 Combination therapy of a serotonin 5-HT1B, 1D receptor agonist, or “triptan” (sumatriptan), and a nonsteroidal anti-inflammatory drug (naproxen sodium) may be beneficial in patients who do not experience relief with monotherapy.3 The average wholesale price (AWP) for nine tablets of the brand product Treximet is about $1,056.4 Because the generic product is still within the first 6 months of exclusivity, nine tablets of the generic version cost $900.4
Dermatologic Agent
Acanya (benzoyl peroxide 2.5%/clindamycin phosphate 1.2%): Acanya is a combination of benzoyl peroxide, an antibacterial and keratolytic agent, and clindamycin, a lincosamide antibacterial.5 This medication is FDA- approved for use in patients aged 12 years or older for the topical treatment of acne vulgaris.5 The combination product of benzoyl peroxide and clindamycin is typically recommended as monotherapy for mild acne. Currently, of the two topical antibiotic options available for the treatment of acne (e.g., clindamycin and erythromycin), clindamycin is preferred because of its superior efficacy against cutaneous staphylococci and Propionibacterium acnes. In order to prevent further increase in resistant strains of P acnes, experts strongly caution never to use topical antibiotics as monotherapy, but always in combination with the bactericidal benzoyl peroxide.6 The generic for Acanya is not yet available; however, Actavis, the current ANDA application holder, was granted a license to market the generic version of the drug beginning July 1, 2018, after reaching a settlement with Valeant Pharmaceuticals International, the brand manufacturer for Acanya.7 The AWP for a 50-g pump is $633.01.4 A pea-sized amount is applied to the face daily, and the product has an expiration date of 10 weeks after dispensing to the patient. (This product is mixed by the pharmacy and expires 10 weeks from the mix date.)5
Urologic Agent
Rapaflo (silodosin): Rapaflo (silodosin) is a uroselective alpha-1 adrenergic receptor antagonist indicated for treatment of benign prostatic hyperplasia (BPH).8 Alpha-blockers are considered preferred medications for the treatment of moderate-to-severe lower urinary tract symptoms caused by BPH.9 Rapaflo, with its uroselectivity, has fewer cardiovascular side effects (e.g., first-dose syncope, orthostatic hypotension, and dizziness) than its nonselective alpha-1 adrenergic counterparts, making a generic alternative appealing. Tamsulosin, the alternative uroselective option on the market, already exists in generic form. Generic silodosin is not yet available to the public; however, Sandoz has an approved ANDA on file with the FDA for the medication as of March 31, 2017. 8 Additionally, the manufacturer of Rapaflo, Kissei Pharmaceuticals, and the U.S.-licensed distributor, Allergan, came to a settlement with Sandoz, dropping its long-standing patent infringement suit in April 2017.10 The AWP for the brand-name drug is $249.27 for a 30-day supply.4
Calcimimetic Agent
Sensipar (cinacalcet): Cinacalcet is FDA-approved for treatment of hypercalcemia in parathyroid cancer, secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (on dialysis), and primary HPT in individuals ineligible for parathyroidectomy.11 Sales of Sensipar, as reported in the Amgen year-end financial results for 2017, totaled $1.374 billion in the U.S. and $1.718 billion worldwide, making the development of a generic alternative a lucrative prospect.12 Generic cinacalcet is not yet available through the two current ANDA application holders, Aurobindo Pharma Ltd. and Cipla Ltd. As the only currently available option, the brand-name medication comes with an AWP ranging from $968.04 (30 mg) to $2,904.12 (90 mg) for a 30-tablet bottle.4 Sensipar may be dosed once daily in primary HPT but is typically dosed twice per day; therefore, the quoted cost would be expected to double.
Anti-Inflammatory Agent
Canasa (mesalamine) rectal suppository: In a study published in 2007 in the Journal of Clinical Gastroenterology and Hepatology and cited by the CDC, the prevalence of ulcerative colitis in the U.S. was found to be 238 cases per 100,000 people.13 Regarding treatment of this debilitating condition, topical mesalamine therapies, such as the Canasa 1-g suppository, are the therapies of choice for mild-to-moderate distal ulcerative colitis as recommended by the Journal of Gasteroenterology guidelines.14 At present, the generic formulation for Canasa is not available on the market. The pharmaceutical company Mylan has an approved ANDA on file with the FDA for the medication (active as of November 24, 2015).15 Additionally, Zydus Pharmaceuticals, a subsidiary of Cadila Healthcare—which did not yet have an approved ANDA at press time— announced a settlement reached with Aptalis, which holds the trademark, to begin marketing its own generic version of Canasa on June 13, 2019.16 Currently, with only the brand name available, the AWP is $1,273.19 for a 30-day supply.4
Chelating Agent
Syprine (trientine hydrochloride): Trientine hydrochloride is a medication used in the treatment of Wilson disease. Approximately 1 in 30,000 people suffer from the condition, which is an inherited autosomal metabolic defect resulting in excess accumulation of dietary copper.17 Buildup of copper over time can lead to life-threatening organ damage, including damage to the brain, kidneys, eyes, and liver. To combat elevated levels of copper, treatment includes a low-copper diet and the use of chelating agents that bind copper and allow for its elimination from the body via the kidneys. Trientine hydrochloride may be preferred by healthcare providers to penicillamine, the alternative chelating agent, owing to an enhanced safety profile.17 On February 9, 2018, Teva Pharmaceutical Industries Ltd. announced the launch of a generic version of Syprine.18 The average cost per 250-mg tablet is $255.20; the initial dose for adults ranges from 750 to 1,250 mg/day. The cost for this medication at 1,250 mg/day for 30 days is $38,280. Teva’s generic is more cost-effective with a price of $36,366 for a 30-day supply of the 1,250 mg/day dosing.4
Multiple Sclerosis Agent
Ampyra (dalfampridine): Dalfampridine is an FDA-approved potassium channel blocker used to improve gait and walking in patients with multiple sclerosis (MS). Dalfampridine was shown to be efficacious in increasing walking speeds in patients with MS.19 Four of the five patents covering Ampyra were invalidated in 2017 by the U.S. District Court, with the fifth patent expiring in July 2018.20 Unless Acorda Therapeutics Inc. wins the appeal for its leading product in early 2018, the generic equivalent for Ampyra is anticipated to be released as early as July 2018.20 According to the FDA website, generic manufacturers Actavis and Aurobindo already have approved ANDAs.15 The AWP for a 30-day supply of the brand product Ampyra is $2,950.4
Antiretroviral Agents
Norvir (ritonavir): The generic equivalent for Norvir, ritonavir, was released by West-Ward Pharmaceuticals Corporation, in March 2018.21 Ritonavir is an HIV protease inhibitor that is FDA-approved for the treatment of HIV-1 infections.22 In HIV treatment, ritonavir is not recommended as monotherapy and must be used in combination with two nucleoside reverse transcriptase inhibitors (NRTIs) and as a “booster” to enhance the pharmacokinetic profile of other protease inhibitors.23 The AWP for a 30-day maintenance supply of the brand product Norvir is a little over $3,700.4
Sustiva (efavirenz): The AB-rated generic for Sustiva, efavirenz, was released in the beginning of 2018. Efavirenz is a nonnucleoside reverse transcriptase inhibitor (NNRTI) that is FDA-approved for combination use with other antiretroviral agents for the treatment of HIV-1 infections.24 In HIV treatment, efavirenz is not recommended as monotherapy and must be used in combination with two NRTIs.23 According to the FDA website, Mylan has 180 days of generic drug exclusivity for the 600-mg tablets, while Aurobindo Pharma has exclusivity on the 50-mg and 200-mg capsules.15 The AWP for a 30-day supply of the brand product Sustiva ranges from about $200 to $1,200 depending on the drug strength.4
Viread (tenofovir disoproxil fumarate): Tenofovir, the generic equivalent for Viread, was released in December 2017 by Teva Pharmaceutical Industries Ltd.25 Tenofovir disoproxil fumarate is an NRTI that is FDA-approved for combination use with other antiretroviral agents for the treatment HIV-1 infections.26 In HIV treatment, tenofovir is not recommended as monotherapy and must be used in combination with another nucleoside NRTI and either an NNRTI or a boosted protease inhibitor.23 In addition, tenofovir is also indicated for the treatment of chronic hepatitis B virus.26 The AWP for a 30-day supply of the brand product Viread ranges from about $1,300 to $1,400 depending on the drug strength.4
Something to consider when discussing cost of antiretrovirals for the treatment of HIV is that they must be used in combination with other antiretroviral agents; consequently, the projected monthly cost is even higher, and the pill burden may be a factor as well with these single agents. Most combination antiretrovirals are still branded products and are relatively expensive.
Phosphodiesterase-5 Enzyme Inhibitors
Adcirca/Cialis (tadalafil): Both Adcirca and Cialis are anticipated to lose product exclusivity in May 2018 and September 2018, respectively.27 The FDA granted United Therapeutics Corporation a 6-month pediatric exclusivity right as Adcirca’s patent was to expire in November 2017.28 Additionally, Eli Lilly and Company entered into a royalty-bearing license agreement with several generic companies that have tentative ANDA approvals, allowing them to release generic products as early as the end of September 2018.29 The generic equivalent for both brand names is tadalafil, a phosphodiesterase-5 enzyme inhibitor. Adcirca dosed at 40 mg daily is FDA-approved for the treatment of pulmonary arterial hypertension; Cialis dosed at 2.5 to 20 mg daily is FDA-approved for the treatment of benign prostatic hyperplasia and/or erectile dysfunction.30,31 The AWP for a 30-day supply of the brand product Adcirca is a little over $4,800. The AWP for a 30-day supply of the brand product Cialis ranges from about $410 to $2,400 depending on the drug strength.4
Levitra/Staxyn (vardenafil hydrochloride): Levitra and Staxyn, both manufactured by Bayer Healthcare Pharmaceuticals Inc., have patents that are expected to expire in October 2018.15 Vardenafil, the generic equivalent for both Levitra and Staxyn, is a phosphodiesterase-5 enzyme inhibitor.32,33 Both products are FDA-approved for the treatment of erectile dysfunction. The difference between Levitra and Staxyn is that the latter is an orally disintegrating tablet (ODT). Levitra is available as 2.5-mg, 5-mg, 10-mg, and 20-mg film-coated tablets; the generic ODT is available as a 10-mg tablet.32,33 The 10-mg film-coated tablet and the 10-mg ODT are not interchangeable, owing to a higher systemic effect provided by the ODT.15 Furthermore, the maximum daily dose for Levitra is 20 mg, while the maximum daily dose for Staxyn is 10 mg.32,33 The AWP for a 30-day supply of the brand product Levitra is a little over $1,800, and Staxyn is about $1,160 for a 30-day supply.4
Antiglaucoma Agent
Travatan Z (travoprost ophthalmic solution): The generic for Travatan Z, travoprost may also be released in 2018. Travoprost is a prostaglandin analogue used in the treatment of glaucoma to lower intraocular pressure.34 Generic manufacturer Argentum Pharmaceuticals LLC initiated a patent cancellation trial against Alcon Laboratories, Inc., at the end of 2017.35 A court decision is expected before the end of 2018. The AWP for a 30-day supply of the brand product Travatan Z is about $410.4
Anticholinergic Agent
Vesicare (solifenacin succinate): The generic equivalent for Vesicare, solifenacin succinate, has the potential to be approved in 2018. This drug is an anticholinergic agent used for the treatment of overactive bladder.36 According to the FDA website, the ANDA for solifenacin succinate submitted by Teva Pharmaceutical Industries Ltd. has tentative approval, and the patent for Vesicare is set to expire in November 2018.15 The AWP for a 30-day supply of the brand product Vesicare is a little more than $410.4
Conclusion
Although for the last several years the FDA has approved ANDAs at record rates, manufacturers of brand-name drugs have employed various strategies to delay the availability of generic-equivalent products. It is possible to anticipate the release of generic substitutions based on patent expiration and tentatively approved applications, but pinpointing the date when the product will be brought to market is challenging.
REFERENCES
1. Association for Accessible Medicines. 2017 annual report. www.accessiblemeds.org/resources/reports/2017-aam-annual-report. Updated 2017. Accessed March 5, 2018.
2. FDA. Office of Generic Drugs. Annual report for 2017. www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/GenericDrugs/ucm567694.htm. Accessed March 5, 2018.
3. Treximet (sumatriptan succinate and naproxen sodium) package insert. Morristown, NJ: Pernix Ireland Limited; May 2015.
4. Red Book Online [database online]. Greenwood Village, CO: Truven Health Analytics. Updated 2018. www.micromedexsolutions.com. Accessed March 18, 2018.
5. Acanya (clindamycin phosphate and benzoyl peroxide gel). Bridgewater, NJ: Valeant Pharmaceuticals International; October 2016.
6. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):73.e33.
7. PR Newswire. Actavis announces Acanya gel patent challenge settlement. www.prnewswire.com/news-releases/actavis-announces-acanya-gel-patent-challenge-settlement-258885451.html. Accessed March 18, 2018.
8. Rapaflo (silodosin capsule) prescribing information. Irvine, CA: Allergan Inc; June 2017.
9. McVary KT, Roehrborn CG, Avins AL, et al. American Urological Association guideline: management of benign prostatic hyperplasia (BPH). 2010, reviewed and validity confirmed 2014. www.auanet.org/education/guidelines/benign-prostatic-hyperplasia.cfm. Accessed March 19, 2018.
10. Eslinger B. Allergan, Kissei settle with Sandoz in prostate drug suit. Law 360. www.law360.com/articles/912499/allergan-kissei-settle-with-sandoz-in-prostate-drug-suit. Accessed March 19, 2018.
11. Sensipar (cinacalcet hydrochloride tablet, coated). Thousand Oaks, CA: Amgen. May 2017. https://Dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45028573-13c4-4c8b-ae62-6c75bba97c81. Accessed March 19, 2019.
12. Amgen. News releases. Amgen reports fourth quarter and full year 2017 financial results. www.amgen.com/media/news-releases/2018/02/amgen-reports-fourth-quarter-and-full-year-2017-financial-results. Accessed March 19, 2018.
13. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424-1429.
14. Kornbluth A, Sachar DB. Erratum: Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):500.
15. Orange Book. Approved drug products with therapeutic equivalence evaluations: Product details for ANDA 204726. www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=A&Appl_No=204726. Accessed March 18, 2018.
16. The Pharmaletter. Zydus settles more US litigation over generic products. www.thepharmaletter.com/article/zydus-settles-more-litigation-in-the-usa-over-generic-products. Accessed March 18, 2018.
17. NIH. National Institute of Diabetes and Digestive and Kidney Diseases. Wilson disease. www.niddk.nih.gov/health-information/liver-disease/wilson-disease. Accessed March 18, 2018.
18. Teva Pharmaceutical Industries Ltd. Latest news. Teva announces launch of a generic version of Syprine® in the United States.
www.tevapharm.com/news/teva_announces_launch_of_a_generic_version_of_syprine_in_the_united_states_02_18.aspx. Accessed March 18, 2018.
19. Goodman AD, Bethoux F, Brown TR, et al. Long-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: results of open-label extensions of two phase 3 clinical trials. Mult Scler. 2015;21(10):1322-1331.
20. GEN. GEN news highlights. Acorda axing 20% of workforce after 4 of 5 Ampyra patents invalidated. www.genengnews.com/gen-news-highlights/acorda-axing-20-of-workforce-after-4-of-5-ampyra-patents-invalidated/81254150. Accessed March 18, 2018.
21. Hikma Pharmaceuticals PLC. Hikma news. Hikma has launched ritonavir tablets USP. www.hikma.com/news/news-article-i2922-hikma-has-launched-ritonavir-tablets-usp. Accessed March 20, 2018.
22. Norvir (ritonavir) package insert. Chicago, IL: AbbVie Inc; June 2017.
23. HHS panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Updated October 17, 2017. www.aidsinfo.nih.gov.uiwtx.idm.oclc.org/ContentFiles/AdultandAdolescentGL.pdf. Accessed March 20, 2018.
24. Sustiva (efavirenz) package insert. Princeton, NJ: Bristol-Myers Squibb Co; October 2017. https://packageinserts.bms.com/pi/pi_sustiva.pdf. Accessed March 20, 2018.
25. Hee Han D. Viread generic now available from Teva. MPR. www.empr.com/generics-news/viread-generic-tenofovir-disoproxil-fumarate-tablets/article/719187. Accessed March 20, 2018.
26. Viread (tenofovir disoproxil fumarate) package insert. Foster City, CA: Gilead Sciences, Inc; August 2012.
27. PR Newswire. Lilly reaches settlement agreement in U.S. Cialis patent ligitation. www.prnewswire.com/news-releases/lilly-reaches-settlement-agreement-in-us-cialis-patent-litigation-300486978.html. Accessed March 17, 2018.
28. Reed T. United Therapeutics gets much-needed reprieve with drug patent. Washington Business Journal. www.bizjournals.com/washington/news/2017/11/21/united-therapeutics-gets-much-needed-reprieve-with.html. Accessed March 17, 2018.
29. Sagonowsky E. Lilly fends off Cialis generics with new patent settlement. Fierce Pharma. www.fiercepharma.com/pharma/lilly-fends-off-cialis-generics-for-another-9-months-new-patent-settlement. Accessed March 17, 2018.
30. Adcirca (tadalafil) package insert. Indianapolis, IN: United Therapeutics Corp; May 2009.
31. Cialis (tadalafil) package insert. Indianapolis, IN: Eli Lilly Co; October 2011.
32. Levitra (vardenafil hydrochloride) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; April 2014.
33. Staxyn (vardenafil hydrochloride) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; June 2010.
34. Travatan Z (travoprost ophthalmic solution) package insert. Fort Worth, TX: Alcon Laboratories, Inc; September 2017.
35. PR Newswire. Argentum Pharmaceuticals succeeds in starting patent cancellation trial against Alcon’s Travatan Z. September 29, 2017. www.prnewswire.com/news-releases/argentum-pharmaceuticals-succeeds-in-starting-patent-cancellation-trial-against-alcons-travatan-z-300528434.html. Accessed March 20, 2018.
36. Vesicare (solifenacin succinate) package insert. Northbrook, IL: Astellas Pharma US, Inc; February 2016.
37. Corporate Pharmacy Services. Upcoming generic drugs. www.corporatepharmacy.com/page/upcoming_generic_drugs. Accessed March 17, 2018.
38. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed March 17, 2018.
39. Pharmacist’s Letter/Prescriber’s Letter. Therapeutic Research Center. PL detail-document #29011: Anticipated availability of first-time generics. January 2018. http://Pharmacistsletter.therapeuticresearch.com/pl/DD_pu.aspx?cs=FACULTY&s=PL&pt=3&dd=290111. Accessed March 17, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.