US Pharm. 2017;42(12):20-26.
ABSTRACT: Irritable bowel syndrome (IBS) is a complex gastrointestinal condition whose pathophysiology is not well understood. Nonpharmacologic treatment options are the mainstay of therapy, and interventions should focus on dietary alterations and physical activity initially and throughout treatment. Drug therapy is rather limited but is focused on the predominant symptoms of IBS—constipation or diarrhea—as well as on improving abdominal pain. Pharmacists have a vital role in patient education and in drug-therapy management for IBS, and they can help ensure that treatment is safe and efficacious.
Irritable bowel syndrome (IBS) encompasses a group of gastrointestinal (GI) conditions that are characterized by pain and abnormal bowel habits that have no identifiable root cause. The pathophysiology of IBS is not completely understood, but a wide variety of factors are believed to be involved. Data are conflicting and have not revealed a specific abnormality that can elucidate why IBS occurs or point to a particular mode of therapy. Many currently used treatments for IBS have addressed GI motility, visceral and dietary sensitivities, inflammation, and alterations in GI flora. One of the more recent etiologies studied is the role of serotonin in the peristaltic reflex of the gut, where an abnormality could lead to increased or decreased motility.1 A 2014 study estimated that 5% to 15% of people worldwide experience IBS symptomatology, making this a disorder that will be encountered often in practice.2
Pathophysiology and Classification
The vague pathophysiology of IBS feeds further into an unclear picture of the disorder’s symptomatology. Generally, IBS is thought to consist of abdominal pain and discomfort, bloating, diarrhea, and constipation.2 While this characterization may be accurate for some patients, IBS may also include psychological and somatic symptoms, such as fatigue, decreased libido, depression, anxiety, and increased daily stress.3 The complexity and diverse scope of IBS have likely contributed to the symptom-management approach generally adopted for patient care.
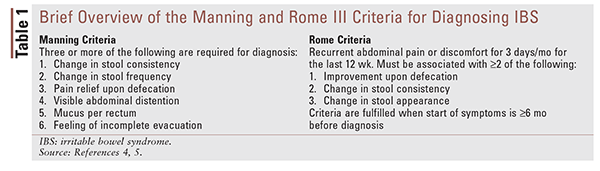
IBS patients present with a wide array of symptoms that can be both specific and nonspecific to the GI tract. Despite this, the two most widely used diagnostic tools for IBS, the Manning criteria and the Rome criteria, consider abdominal pain and abnormal bowel habits to be principal criteria.4,5 For a brief overview of these diagnostic tools, see TABLE 1.
In IBS, abdominal pain can present in several different ways. Patients experience varying degrees of pain, ranging from mild discomfort to severe, debilitating exacerbations.4,5 There may be alleviating behaviors, such as defecation, and stress and eating may worsen the pain. Diarrhea secondary to IBS usually is frequent and is small-to-moderate in volume.4,5 Patients are more likely to experience these bouts postprandially or early in the morning. More than one-half of patients who experience diarrhea linked to IBS report a mucous discharge in the stool.3 Constipation, much like abdominal pain, is variable and can present in different forms and severities among patients. Feelings of incomplete evacuation are common in all types of IBS.3
Because of the diverse presentations of diarrhea and constipation in patients, IBS is categorized into several subtypes: IBS with predominant constipation (IBS-C), IBS with predominant diarrhea (IBS-D), and mixed or alternating IBS (IBS-A). The fourth subtype, postinfectious IBS, is associated with a GI infection and is uncommon.3 This review will discuss the management of IBS-C and IBS-D.
Since IBS may present dramatically differently from patient to patient, diagnosis should made only when all other etiologies have been ruled out. Initially, physical examinations and routine laboratory tests should be conducted. Patients presenting with diarrhea should be screened for celiac disease, monitored for malabsorption, and considered for endoscopy; in rare cases, a stool culture should be collected. If a diagnosis of constipation is unclear, radiography may be used to detect retained stool, and endoscopy is likely warranted to inspect for structural damage. If the patient appears to have one of the other IBS subtypes, the procedures performed to rule out other causes should be personalized to the patient. Some symptoms should signal the provider that the diagnosis is likely not IBS, such as bloody stools, anorexia, malnutrition, weight loss, pain that restricts or alters sleep habits, and greasy stools.1,3 These symptoms should prompt further investigation into other causative factors.
Initial Treatment
The majority of pharmacologic options for IBS patients treat symptoms, rather than correct an underlying cause. Therefore, it is advantageous to make lifestyle and dietary modifications initially.3 In more severe cases, or in patients who continue to experience symptoms despite these modifications, pharmacotherapy may be warranted.
Physical activity should be recommended based on the patient’s characteristics and capabilities. In a 2011 study that examined the effects of getting 20 to 60 minutes of daily exercise on GI symptoms, there was a significant improvement in symptoms and overall disease management in physically active patients.6 In any event, increased activity is unlikely to exacerbate symptoms.
Modification of dietary habits should also be a first-line consideration. Avoidance of foods that are gas-producing and high in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (i.e., FODMAPs) and that have a history of aggravating symptoms may provide benefit. A 2015 study showed that both dietary advice and adherence to an IBS diet helped reduce symptoms of IBS.7 Monitoring diet and symptoms may also help identify problematic foods. One food that may be beneficial or harmful, depending on the type consumed, is fiber. Fiber has been considered a mainstay of IBS therapy despite limited evidence supporting its use. Insoluble fiber is more likely to cause symptoms, whereas soluble fiber, such as psyllium, may provide benefit. Gluten sensitivities and celiac disease have been linked to IBS.8 Although data show that dietary interventions may be effective, inconsistent findings on specific foods, as well as the patient’s individual preferences, may make this treatment option challenging to implement. Guidelines encourage an individualized approach and provide little additional guidance.9
Adjunctive Treatment of IBS-D
Antidiarrheal Agents: Loperamide, the agent most commonly used to treat diarrhea, acts on opioid receptors to slow intestinal transit time and stop peristalsis.10 It also alters stool formation by decreasing stool volume and stopping fluid loss.11 Eluxadoline is another agent approved for IBS-D. This medication works on the delta-opioid receptor as an antagonist and on kappa and mu-receptors as an agonist.12 The most common adverse effects (AEs) of these agents are dizziness, fatigue, constipation, and abdominal pain.11 In general, guidelines suggest that loperamide is better than no therapy, but it has not demonstrated global benefit.13 Eluxadoline’s place in therapy is not well understood, and its cost may preclude its use in some patients.
5-Hydroxytryptamine3 (5-HT3) Receptor Antagonist: Alosetron is a 5-HT3 receptor antagonist that acts on ion channels throughout the GI tract.14 These channels control pain perception, colonic transit, and secretions. Alosetron has some severe AEs, including ischemic colitis, and it was pulled from the market in 2000.11 It is now available only through restricted prescribing programs and is limited to women who have had symptoms for at least 6 months and have received no benefit from other therapies.13 The dosage, which is 0.5 mg twice daily for 4 weeks, may be increased to 1 mg twice daily if the response is inadequate.11 Guidelines recommend that alosetron be considered for women with IBS-D, but its limited evidence and moderate benefit render it a last-line option.13
Rifaximin: Antibiotic use in IBS has been a point of controversy, especially given the greater emphasis being placed on stewardship. Rifaximin binds to DNA-dependent RNA polymerase, thereby blocking bacterial RNA synthesis.15 The recommended dosage is 550 mg three times daily for 14 days.11 In general, rifaximin is used primarily in patients who do not have IBS-C and are experiencing GI symptoms such as abdominal pain and bloating. Guidelines suggest that antimicrobial treatment with rifaximin is better than no treatment and that it may benefit global disease management.13 It is important to note that no evidence supports repetitive treatments and that cost may prohibit treatment. Important AEs are peripheral edema, dizziness, fatigue, ascites, and, possibly, headache and depression.11 Rifaximin should be recommended only in refractory cases of IBS-D.
Adjunctive Treatment of IBS-C
Lubiprostone: Lubiprostone works by activating chloride channels in the GI tract to increase the amount of fluid secreted into the stool, which in turn increases transit. AEs include headache, nausea, edema, dizziness, and GI upset.11 The dosage is 8 mcg twice daily, which, notably, is far less than the dosage of 24 mcg twice daily recommended for chronic idiopathic constipation.11,15 For IBS-C, lubiprostone is indicated only in female patients aged 18 years and older.9 Based on evidence of encouraging outcomes, guidelines generally recommend lubiprostone in the treatment of IBS-C. Several studies have found lubiprostone to be more effective than placebo, although it has never been studied head-to-head against other agents used for IBS-C.13 This medication is more expensive, and higher out-of-pocket expenses should be expected.
Guanylate Cyclase Agonists: Linaclotide and its metabolite work as agonists to guanylate cyclase-C on the intestinal epithelium, causing an increase in cyclic guanine monophosphate (cGMP).9 The rise in cGMP leads to chloride and bicarbonate secretion into the lumen, decreasing transit time. Extracellular cGMP is also increased and may work to lessen visceral pain by reducing pain-receptor activity. The dosage for IBS-C is 290 mcg per day, and the primary AE is diarrhea, which may be severe.11 Outcomes in studies of linaclotide have been positive, and most patients experience at least a moderate benefit; however, cost may be a concern for some patients and may limit its accessibility compared with less expensive options.
Polyethylene Glycol (PEG): PEG is a widely used and well-tolerated osmotic laxative that works by enabling water retention in the stool.16 The usual dosage is 17 g mixed in 4 to 8 oz of a beverage.11 Generally, AEs such as abdominal cramps, nausea, bloating, diarrhea, and flatulence limit consistent use. Guidelines do not support PEG use in IBS, based on low-quality evidence and lack of symptom relief.13
5-Hydroxytryptamine4 (5-HT4) Receptor Agonists: Tegaserod is a selective 5-HT4 agonist that acts in the GI tract to enhance secretion, increase peristaltic movement, and increase transit.9 Tegaserod was approved in 2002 but was later removed from the market because of cardiovascular AEs. Different 5-HT4 antagonists are marketed in other countries, but none are approved in the United States.
Adjunctive Treatments for Abdominal Pain
Antispasmodics: Hyoscyamine, pinaverium, mebeverine, and dicyclomine hydrochloride are antispasmodics that have shown benefits in IBS.9 Evidence regarding antispasmodics is weak and extremely limited, which should be kept in mind in recommending these agents. If an antispasmodic is prescribed, short-term or as-needed use is recommended, and it should not be considered a long-term solution.6 Antispasmodics used in IBS work either by direct relaxation of the intestinal smooth muscle or by their anticholinergic or antimuscarinic properties.11 Guidelines address the importance of antispasmodic agents and their role in IBS therapy, but most studies are outdated or methodologically flawed, and some of the agents investigated are no longer available in the U.S.9,13 The limiting AEs of these medications are generally anticholinergic, and agent choice will likely be affected by prescriber preference.
Antidepressants: Both tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been shown beneficial for global IBS symptoms and pain relief. Most research on antidepressants in IBS patients has focused on TCAs, likely because of anticholinergic effects that help slow transit time in IBS-D, along with mood-improving effects.9 Options are amitriptyline, nortriptyline, imipramine, and desipramine.9 Important AEs of TCAs are QT prolongation and anticholinergic effects, such as constipation and sedation.11 Data on SSRIs and SNRIs are controversial.17 SSRIs and SNRIs should be considered in a patient with IBS-C who also has depressive symptoms or a concomitant diagnosis of depression.
Probiotics and Alternative Therapies: Many patients have tried OTC products and alternative treatments that may or may not be supported by evidence. Probiotics are often considered by IBS patients, but based on a lack of evidence and knowledge about which strains are effective, guidelines do not support their use.9,18 Peppermint oil has demonstrated antispasmodic effects and may be effective for visceral pain, but evidence is limited and controversial.13,19 Mindfulness training, cognitive behavioral therapy, and other complementary and alternative medicine options exist; however, evidence is limited, and these therapies should be recommended cautiously.8,9 Although these treatments are unlikely to pose much risk, other options are more efficacious.
Role of the Pharmacist
The complexity of IBS renders frequent monitoring and therapy adjustments necessary in order to provide effective patient care. Although pharmacologic therapy should not be recommended as first-line treatment, pharmacists can serve as part of the patient-support system and must be prepared to offer recommendations should adjunctive therapy be needed. By advising against therapies that do not have evidence to support use and identifying symptoms that can be effectively treated, pharmacists can be an integral part of the care team and an asset to the primary care provider in managing drug therapy. Whether in the hospital or a community setting, the pharmacist should reconcile medication lists to make therapy recommendations that can help improve disease management and improve quality of life.
Conclusion
IBS is a complex disease with a pathophysiology that is poorly understood. With its wide array of symptoms ranging from constipation to diarrhea and a multitude of possible presentations, a one-size-fits-all approach to treatment is inappropriate for most patients. Instead, the initial treatment approach for IBS should involve nonpharmacologic management and then focus on drug therapy for the individual patient’s predominant symptoms according to the limited evidence-based medicine supporting specific agents in the treatment of IBS symptomatology. The monitoring, education, and support of patients that pharmacists provides renders their role vital in IBS management.
REFERENCES
1. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773.
2. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
3. Occhipinti K, Smith JW. Irritable bowel syndrome: a review and update. Clin Colon Rectal Surg. 2012;25:46-52.
4. Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653-654.
5. Ford AC, Bercik P, Morgan DG, et al. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262-1270.
6. Johannesson E, Simrén M, Strid H, et al. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106:915-922.
7. Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399.
8. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
9. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
10. Lavö B, Stenstam M, Nielsen AL. Loperamide in treatment of irritable bowel syndrome—a double-blind placebo controlled study. Scand J Gastroenterol Suppl. 1987;130:77-80.
11. Lexicomp Online. Lexi-Drugs, Hudson, OH: Lexi-Comp, Inc; 2017. http://online.lexi.com. Accessed August 30, 2017.
12. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
13. Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1149-1172.
14. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
15. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
16. Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol. 2013;108:1508-1515.
17. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367-378.
18. Ford AC, Quigley EM, Lacy BE, et al. Effect of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
19. Cappello G, Spezzaferro M, Grossi L, et al. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530-536.
To comment on this article, contact rdavidson@uspharmacist.com.






