US Pharm. 2019;44(2):47-48.
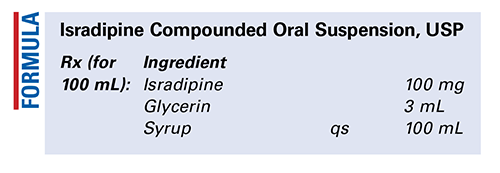
Method of Preparation: Calculate the quantity of each ingredient needed. Accurately weigh or measure each ingredient. The isradipine may be obtained as either powder or capsules; if the capsules are used, empty the required number into a suitable mortar, or use isradipine powder. Add the glycerin to wet the powder and triturate to a fine paste. Add the syrup in small portions geometrically to make the isradipine pourable. Transfer the contents of the mortar, stepwise and quantitatively, to a calibrated bottle. Add enough of the syrup to bring to final volume, and mix well. Package and label.
Use: Isradipine is used in the management of hypertension to reduce the risk of stroke and heart attack, and it may be used alone or concurrently with thiazide-type diuretics.
Packaging: Package in tight, light-resistant, and child-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. Store in a refrigerator. Shake well before taking.
Stability: A beyond-use date of not more than 30 days after the date on which it was compounded, when stored in a refrigerator, may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH (5.5-6.5), specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Isradipine Compounded Oral Suspension USP is one of the official monographed compounded preparations in the USP. Accordingly, the monograph has a preparation definition, assay, specific tests (pH), and additional requirements (Packaging and Storage, Beyond-Use Date, Labeling, and USP Reference Standard).3
Isradipine, a calcium antagonist, is commercially available only as 2.5-mg or 5-mg capsules. It occurs as a yellow, fine, crystalline powder that is odorless or has a faint characteristic odor. Isradipine is practically insoluble in water (<10 mg/L at 37°C), but it is soluble in ethanol and freely soluble in acetone, chloroform, and methylene chloride. Inactive ingredients in the capsules include colloidal silicon dioxide, D&C Red No. 7 Calcium Lake, FD&C Red No. 40 (5-mg capsule only), FD&C Yellow No. 6 Aluminum Lake, gelatin, lactose, starch (corn), titanium dioxide, and other ingredients. The capsules may also contain benzyl alcohol, butylparaben, edetate calcium disodium, methylparaben, propylparaben, and sodium propionate. The capsules should be stored at room temperature and dispensed in a tight, light-resistant container.3
Glycerin (glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two-thirds as sweet as sucrose. Glycerin is used as an antimicrobial preservative, emollient, and humectant; in ophthalmic formulations; as a plasticizer in film coating for tablets; as a parenteral solvent; and as a sweetening agent in alcoholic elixirs (up to 20% concentration). Glycerin has a specific gravity of about 1.25 and a melting point of 17.8°C; if cooled to crystallization, it must be heated to about 20°C to melt. Glycerin is miscible with water, methanol, and 95% ethanol; practically insoluble in oils and chloroform; and slightly soluble in acetone. It is hygroscopic and should be stored in airtight containers in a cool place. Glycerin is not prone to oxidation, but it will decompose upon heating. When it is mixed with water, ethanol, and propylene glycol, the mixtures are chemically stable. Incompatibilities include strong oxidizing agents that may cause it to explode, such as chromium trioxide, potassium chlorate, and potassium permanganate. When mixed with zinc oxide or basic bismuth nitrate and exposed to light, glycerin will form a black discoloration. When glycerin is mixed with phenols, salicylates, or tannin, a darkening of the mixtures may occur, owing to an iron contaminant in the glycerin. It will also form a strong acid complex, glyceroboric acid, when it is mixed with boric acid.4
Syrup (simple syrup) is a clear, sweet vehicle that is used as a sweetening agent and as the base for many flavored and medicated syrups. It contains 85% w/v sucrose in water and has a specific gravity of not less than 1.30. Syrup is generally self-preserving as long as the sucrose concentration is maintained sufficiently high. It is best to prepare syrup without the use of heat; however it may also be prepared with boiling water. Syrup should be stored in tight containers, preferably in a cool place.1
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; January 2019.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. RxList. Dynacirc. www.rxlist.com/dynacirc-drug.htm. Accessed January 7, 2019.
4. Alvarez-Nunez FA, Daurio D. Glycerin. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:401-405.
To comment on this article, contact rdavidson@uspharmacist.com.






