US Pharm. 2018;43(4):HS2-HS6.
ABSTRACT: Leptospirosis is a bacterial infection that is emerging as a public-health concern with significant impact around the world. This infection has previously been underrecognized and underreported; however, awareness is slowly increasing given the leptospirosis outbreaks that have followed recent heavy rains and hurricanes. The infection is transmitted by host animals to humans through contaminated food and water. The use of protective gear and sanitation precautions can help minimize the risk of infection. Symptoms range from mild and nonspecific to serious and fatal. Prophylaxis, early diagnosis, and antibiotic treatment are essential for preventing severe complications, as well as death, from leptospirosis.
Leptospirosis, the most prevalent and widespread zoonotic disease in the world, is a leptospiral (spirochete) infection that occurs in temperate and tropical environments.1,2 Transmission takes place via the urine or feces of infected animals, and humans become infected by ingesting contaminated food or water.3 The bacteria can enter the body through portals of cuts and abrasions, the mucous membranes, or the conjunctiva of the eyes; however, they are not thought to penetrate intact skin. Leptospirosis is both an occupational and a recreational hazard, and the bacteria can survive in water or soil for weeks to months.3 Outbreaks are often perpetuated by changing environmental trends, especially the hurricanes and the increased rainfall and flooding that occur more frequently in warmer regions.4
Epidemiology
According to the World Health Organization, the incidence of leptospirosis in tropical regions is 10 times higher than in temperate climates.5 It is estimated that, each year, more than one million cases occur worldwide that result in 58,900 deaths.6 This disease was considered to be limited to the tropics and rural regions, but there has been an increase of cases in urban areas of industrialized and developed countries as well.1 Recent outbreaks have been reported in popular travel destinations, including the Caribbean and Latin America, the Indian subcontinent, Southeast Asia, Oceania, and some parts of Eastern Europe.7 Of the 100 to 200 cases identified each year in the United States, half occur in Hawaii.8 However, the actual incidence in Hawaii is estimated to be double what has been reported.4
The true incidence of leptospirosis is unknown owing to underreporting and insufficient understanding of the disease. The clinical signs and symptoms are difficult to differentiate from those of other endemic diseases. Reporting is also limited because of the lack of access to appropriate diagnostic laboratory services in high-risk areas.4 Because many of the signs and symptoms of leptospirosis are virtually the same as those of dengue, leptospirosis has often been misdiagnosed.
In Puerto Rico, for example, leptospirosis was rarely reported despite the occurrence of 208 cases between 1948 and 1952. After Hurricane Hortense in 1996, heavy rainfall and flooding swept the island. Dengue was initially suspected; however, serum results were negative for dengue but positive for Leptospira-specific immunoglobulin M (IgM) antibodies. The dengue-based screening was performed across the island, and laboratory-confirmed cases of leptospirosis increased from 6% before the hurricane to 24% after it.9
Natural disasters are associated with a greater number of reported cases because of the increased vulnerability and sanitation concerns of the affected population. Notable outbreaks that were attributable to natural disasters include flooding in Nicaragua (1995), a cyclone in Orissa, India (1999), flooding in Mumbai, India (2000, 2005) and Jakarta, Indonesia (2002), a typhoon in the Philippines (2009), and hurricanes in Puerto Rico and the Dominican Republic (2017).5,10,11 Leptospirosis is not yet recognized as a public-health threat, but it does have emerging potential as an epidemic that could impact human health worldwide.5
Microbiology
Leptospirosis is an infectious disease caused by the pathogenic genus Leptospira. This species of spirochetes has a tightly coiled lipopolysaccharide (LPS) outer membrane that is similar to that of other gram-negative bacteria, allowing them to survive in diverse environments. Leptospires are obligate aerobes with filaments (periplasmic flagella) that enable motility in liquid media such as blood, urine, and cerebrospinal fluid, where they commonly reside.1,12 The optimal growth temperature for Leptospira is 28°C to 30°C.1,12 Direct visualization is difficult because leptospires Gram stain poorly, instead requiring dark-field or phase-contrast microscopy.1 These bacteria, which are distinguished by the hook at one or both ends, are able to pass through 0.45-µm filters.1,12
This LPS is responsible for the antigenic differences dividing Leptospira into serovars (serotypes) that are associated with the various reservoir hosts. The infection of specific serovars depends on which animal carriers (e.g., mongoose, rat) are present in the human population.1,13 There are 21 species of Leptospira, nine of which are considered pathogenic.
Transmission
About 160 mammalian species, both wild and domestic, carry pathogenic leptospires in the kidneys and genital tract.2,3,14 Common reservoirs include cattle, horses, pigs, dogs, and rodents that are typically asymptomatic for the disease.3 Rodents, especially the brown rat (Rattus norvegicus), are recognized as the primary source of infection in humans.15 Natural hosts have a lifelong infectious period, whereas accidental hosts are infectious for days or months.2 In dogs, leptospirosis caused by Leptospira interrogans should be strongly suspected as part of a differential diagnosis if the sclera of a dog’s eyes appear jaundiced (even slightly so), indicating liver damage. Human-to-human transmission is rare but may occur via sexual contact and breastfeeding.2,14
The bacterium enters the environment (i.e., water and soil) through urine excretion. Leptospires prefer warm, humid environments, resulting in a higher incidence of infection in tropical climates during the rainy season.12 As noted earlier, bacteria can be transmitted through skin abrasions and mucous membranes of the eyes, nose, and mouth.14 Some populations have an increased risk of exposure because of their occupation or lifestyle. Higher-risk occupations include farmers, agricultural workers, trappers, sewer workers, slaughterhouse workers, veterinarians, fish workers, dairy farmers, and military personnel. Travelers are also at increased risk when they participate in outdoor water-related activities such as swimming, kayaking, and rafting in tropical climates. Wading in contaminated floodwaters can lead to outbreaks within local communities.3 There is an increased risk of household exposure from pet dogs, domesticated livestock, or rodent infestation.
Clinical Presentation
Leptospirosis may present with varying symptom severity, from mild and without obvious symptoms to fatal sequelae. The infection may occur in two phases. In the first phase, patients typically have nonspecific symptoms such as high fever, headache, chills, muscle pain, cough, nausea, vomiting, diarrhea, and rash. Patients may also present with jaundice, red eyes, or abdominal pain.3,8,14,15 The incubation period ranges from 2 to 30 days, but symptoms arise abruptly during the first phase of the infection.8,14 Patients typically recover within a few months without treatment but may fall ill again, leading to the second phase.1 This phase is potentially fatal given that some patients present with more severe symptoms, such as organ failure and meningitis.3,8
The combination of kidney failure and liver failure is one of the most clinically recognizable signs of leptospirosis, and this severe form of the disease is known as Weil’s disease.15 In some cases, abnormalities in coagulation lead to severe pulmonary hemorrhage, which is fatal in more than 50% of cases.15 Severe outcomes depend on patient age (>60 years), environment, host susceptibility, and virulence of the bacterial species.15
Diagnosis
Diagnosis may be difficult because the nonspecific symptoms of leptospirosis are often mistaken for influenza, dengue, typhoid, or viral hepatitis, which are endemic in many parts of the world.14 Patients with suspected leptospirosis should be referred to a healthcare provider for appropriate laboratory testing and swift administration of treatment.
Detection of leptospires may occur on a medium after 3 to 4 days and be subcultured after 7 to 21 days, despite survival in liquid culture for months to years.1 Detection may require an incubation period of 1 to 6 weeks.13 For rapid results, microscopic agglutination or polymerase chain reaction (PCR) testing may be used to detect Leptospira-specific IgM antibodies in serum samples.16 Results may be detectable by day 6 of illness and for up to 2 to 3 months after symptom onset.1,13 Serologic testing may be used to determine genus and serovar, but it cannot provide definitive results owing to the prolonged elevation of antibodies.13,15 Urine cultures may be used for diagnostic testing, but urine samples should not be obtained until after the second week of symptoms because of the variance in duration of urinary excretion and limited survival of leptospires in urine.15 Molecular techniques are available that use real-time PCR and loop-mediated isothermal amplification, which has a high specificity for the diagnosis of leptospirosis.17 Disease identification relies heavily on patient risk factors, exposure, and signs and symptoms. Therefore, negative diagnostic results should not rule out a possible infection.
Treatment
The management of leptospirosis is dependent on the severity of symptoms. Mild cases typically self-resolve; however, antimicrobial therapy may prevent progression to the second phase of the disease. With treatment, the recovery time ranges from 3 days to a few weeks.3
Susceptibility testing is sometimes difficult owing to the required media, long incubation period, and contamination of urine samples.15 Therefore, empiric therapy is recommended when infection is suspected, especially in severe cases.
There are no FDA-approved drugs for the treatment of leptospirosis, although most antibiotics that are used are designated as off-label treatment. A number of published studies support the use of a limited group of antibiotics that have been shown to be effective (TABLE 1).
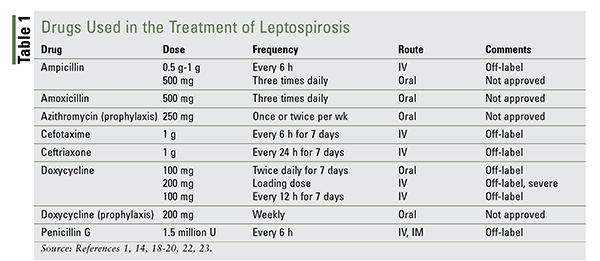
Four drugs are used orally to treat mild cases: ampicillin, amoxicillin, azithromycin, and doxycycline. Amoxicillin may also be considered for pregnant patients.
A clinical study by McClain and colleagues demonstrated that doxycycline use resulted in a shortened duration of infection, symptom improvement, and prevention of bacterial shedding in the urine.18 Hospitalized patients with severe disease have traditionally been treated with IV penicillin G, although IV doxycycline and ceftriaxone are commonly used. One study that compared IV penicillin G with ceftriaxone found that their efficacy and cost were equal, although ease of administration and greater antibacterial coverage shifted the recommendation to ceftriaxone.19 It should be noted that penicillin and cephalosporins lack activity against Rickettsia (another spirochete) and should be avoided in circumstances where leptospirosis cannot be definitively distinguished from a Rickettsia infection. Doxycycline is appropriate therapy in these patients.20
Another concern is the development of a Jarisch-Herxheimer reaction, which may occur following the treatment of antimicrobial therapy for leptospirosis. This is an acute inflammatory response to the clearance of spirochetes from the circulation that is characterized by fever, rigor, and hypotension.21
Along with antibiotics, supportive therapy is necessary owing to the risk of renal and hepatic dysfunction. Patients may need hydration and potassium supplementation, and some may require more aggressive treatment, such as hemodialysis. Patients experiencing pulmonary hemorrhage or respiratory failure should be treated with corticosteroids and ventilation.15
Prevention
Reducing exposure to risk factors is essential for appropriate prevention. Persons in at-risk occupations should minimize contact with possibly contaminated environments by wearing protective clothing such as boots, gloves, aprons, and masks. Travelers and locals should be forewarned about contaminated bodies of water, such as ponds, pools, rivers, or streams.14 Submerging one’s head under water while swimming increases the exposure risk by transferral through mucous membranes of the eyes, nose, and mouth.8 Skin lesions or abrasions should be covered with waterproof dressings. If a person is exposed to contaminated water or urine, it is recommended to wash or shower immediately thereafter.14
To prevent further spreading within the community or household, rodent problems should be vigorously controlled. Vaccinations are available for farm animals and pets, but they do not cover all strains. Although most animals do not exhibit signs or symptoms of leptospirosis, they serve as a common source of human contamination. Farmers and pet owners should take precautions if their animals are infected and should refrain from handling contaminated urine, blood, or tissues. Cleanliness and sanitation are mainstays of leptospirosis prevention. Frequent washing of hands after handling excrement or urine and cleaning surfaces with an antibacterial cleaning solution are also recommended. A homemade solution with one part bleach to 10 parts water may be used.3 Antibiotics may be prescribed for pets, but this is done more for the owner’s protection. Vaccinations in humans during leptospirosis outbreaks have shown varying results and are still being studied.1,12,15 Prophylactic antibiotics for humans during leptospirosis outbreaks have shown some efficacy.22
The Pharmacist’s Role
The accessibility of the pharmacist provides ample opportunity to promote awareness of leptospirosis in patients and members of the healthcare team. Travelers often frequent their pharmacy before going abroad or on vacation, providing an opportune time for prevention education and counseling. Pharmacists should consider recommending antibiotic prophylaxis for persons who are traveling to high-risk locations. Education is also beneficial during seasonal changes, when flooding is more common. Early identification and treatment of leptospirosis are important to reduce disease severity and improve prognosis. Pharmacists should be readily available to provide appropriate pharmacologic treatment, counseling, and supportive therapy to their patients.
REFERENCES
1. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757-771.
2. European Centre for Disease Prevention and Control. Factsheet about leptospirosis. https://ecdc.europa.eu/en/leptospirosis/factsheet. Accessed October 28, 2017.
3. CDC. Leptospirosis. www.cdc.gov/leptospirosis/index.html. Accessed October 22, 2017.
4. World Health Organization. Leptospirosis Burden Epidemiology Reference Group (LERG). www.who.int/zoonoses/diseases/lerg/en/index2.html. Accessed October 28, 2017.
5. Schneider MC, Jancloes M, Buss DF, et al. Leptospirosis: a silent epidemic disease. Int J Environ Res Public Health. 2013;10:7229-7234.
6. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898.
7. Pappas G, Papadimitriou P, Siozopoulou V, et al. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008;12:351-357.
8. State of Hawaii Department of Health Disease Outbreak Control Division. Leptospirosis. http://health.hawaii.gov/docd/disease_listing/leptospirosis. Accessed October 29, 2017.
9. Sanders EJ, Rigau-Pérez JG, Smits HL, et al. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966]. Am J Trop Med Hyg. 1999;61:399-404.
10. Nedelman M. Suspected leptospirosis cases increasing in Puerto Rico after Hurricane Maria. www.cnn.com/2017/10/24/health/leptospirosis-puerto-rico/index.html. Accessed October 25, 2017.
11. GardaWorld. Dominican Republic: leptospirosis leaves at least 52 dead this year. November 3, 2017. www.garda.com/crisis24/news-alerts/77061/dominican-republic-leptospirosis-leaves-at-least-52-dead-this-year. Accessed on February 26, 2018.
12. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296-326.
13. Mayo Medical Laboratories. Leptospira, IgM, serum. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/65183. Accessed October 30, 2017.
14. World Health Organization. Leptospirosis. www.wpro.who.int/mediacentre/factsheets/fs_13082012_leptospirosis/en. Accessed October 22, 2017.
15. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
16. Frawley AA, Schafer IJ, Galloway R, et al. Notes from the field: postflooding leptospirosis – Louisiana, 2016. Morb Mortal Wkly Rep. 2017;16:1158-1159.
17. Picardeau M, Bertherat E, Jancloes M, et al. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagn Microbiol Infect Dis. 2014;78:1-8.
18. McClain JB, Ballou WR, Harrison SM, Steinweg DL. Doxycycline therapy for leptospirosis. Ann Intern Med. 1984;100:696-698.
19. Panaphut T, Domrongkitchaiporn S, Vibhagool A, et al. Ceftriaxone compared with sodium penicillin G for treatment of severe leptospirosis. Clin Infect Dis.2003;36:1507-1513.
20. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39:1417-1424.
21. Guerrier G, Lefèvre P, Chouvin C, D’Ortenzio E. Jarisch-Herxheimer reaction among patients with leptospirosis: incidence and risk factors. Am J Trop Med Hyg. 2017;96:791-794.
22. Takafuji ET, Kirkpatrick JW, Miller RN, et al. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl J Med. 1984;310:497-500.
23. Hartskeerl RA, Wagenaar JP. Leptospirosis. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2014.
To comment on this article, contact rdavidson@uspharmacist.com.






