US Pharm. 2018;43(4):27-32.
ABSTRACT: Helicobacter pylori infection is prevalent in about one-half of the world’s population. Infection with H pylori is associated with the development of peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue lymphoma. Significant scientific advances have been made in the management of H pylori infection in adults; these advances are addressed in updated recommendations by the American College of Gastroenterology and the Toronto Consensus. The increasing prevalence of H pylori that is resistant to traditional clarithromycin-based therapies is a global problem requiring a review of the evidence to incorporate additional regimens.
Helicobacter pylori is a gram-negative bacterium that colonizes the human stomach. It is typically acquired during childhood and transmitted from human to human.1,2 Risk factors for H pylori infection include low socioeconomic status, increasing number of siblings, and having an infected parent.1
Nearly one-half of the world’s population is infected with H pylori. The significant global prevalence and increasing antibiotic resistance led the World Health Organization to recognize H pylori as a high-priority pathogen in 2017. The prevalence of H pylori varies by geographic region, and it is estimated that 30% of the U.S. population is infected.1,3,4 H pylori is a major pathogen that causes gastrointestinal diseases such as peptic ulcer disease (PUD), gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma, and it is associated with nongastric diseases including iron-deficiency anemia (IDA) and idiopathic thrombocytopenic purpura (ITP).5,6 Updated U.S. and Canadian recommendations incorporate the latest evidence on managing H pylori infections.
Diagnosis
Historically, H pylori testing was indicated for patients with clinical manifestations of H pylori infection, including a history of or active PUD or MALT lymphoma.1,2,5 Recent evidence has contributed to the expansion of testing to include patient groups that previously were considered controversial. In addition to gastrointestinal manifestations of H pylori infection, evidence also supports the use of H pylori testing in nongastric diseases such as unexplained IDA and ITP.1,2,5 See TABLE 1.

Tests for H pylori are categorized as nonendoscopic or endoscopic (TABLE 2). Considerations for selecting a diagnostic test include the ability to perform the test, clinical situation, and cost. Although there is no gold-standard test for identifying H pylori, the urea breath test (UBT) is preferred in patients without alarm symptoms because it is noninvasive, inexpensive, and highly sensitive and specific.2 Culture, another method for diagnosing H pylori, allows for susceptibility testing; however, it is not widely available in the U.S. Culture and susceptibility testing should be considered in areas of high clarithromycin resistance or when therapy has failed, because failure of a first-line regimen indicates a 60% to 70% chance of resistance.
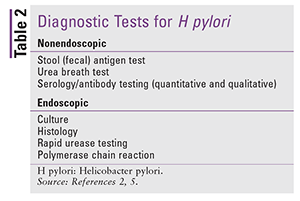
Pharmacists should be aware of medications that can affect the different types of tests.2,5 To avoid false-negative results with the rapid urease test, UBT, or stool antigen test (SAT), proton pump inhibitors (PPIs) should be discontinued at least 2 weeks prior to testing, and bismuth and antibiotics should be withheld at least 4 weeks prior. H2 receptor antagonists have minimal effect on the sensitivity of diagnostic tests for H pylori, and there is no recommendation to withhold them prior to testing. Antacids have no effect on diagnostic tests and could be used for symptom relief while PPI therapy is withheld.2
Treatment
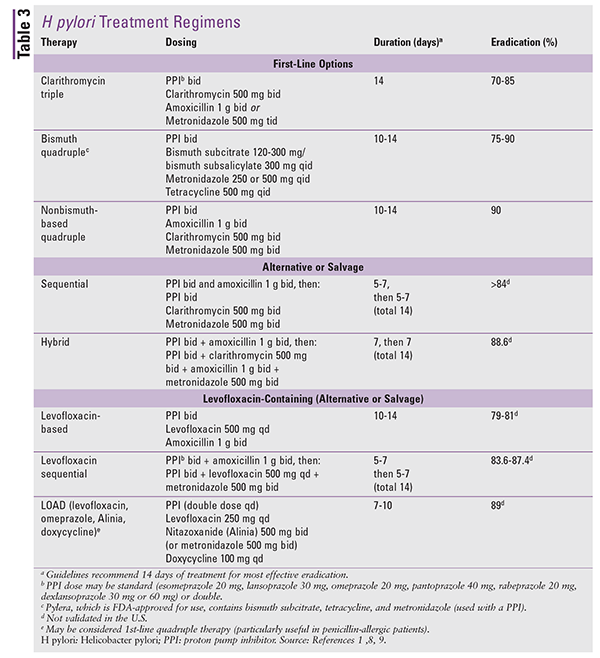
H pylori treatment should be prescribed to all patients with a positive test of active infection, and the selected treatment regimen should provide an eradication rate of at least 90%. H pylori regimens include triple therapy, sequential therapy (patient is given one treatment, followed by another), quadruple therapy, and levofloxacin-based triple therapy (TABLE 3).7 In the selection of the most appropriate empiric treatment regimen for H pylori, previous antibiotic exposure, regional antibiotic-resistance patterns, and eradication rates should be taken into consideration because these factors can impact successful treatment. Successful treatment also relies on host factors such as allergies and adherence.8-10
First-Line Regimens
Two triple-therapy regimens considered to be first-line contain clarithromycin, a PPI, and either amoxicillin or metronidazole.1,7-9,11 Clarithromycin-based therapies were considered to be the best-tolerated and safest; however, increasing rates of antibiotic resistance have led to reduced efficacy.12,13 Eradication rates with clarithromycin-based therapy are below 80%; therefore, this therapy should be reserved for geographic areas where resistance to clarithromycin is less than 15% and for patients without previous macrolide exposure.1,7-9,12
H pylori’s mechanism of resistance to clarithromycin is being investigated but is thought to be attributable to two major mutations (A2142G and A2143G) in the peptidyl transferase region encoded in domain V of 23S rRNA.6,13 Current findings also suggest that at least four efflux-pump families of gene clusters in H pylori strains can have synergistic effects that induce antibiotic resistance.13 Additional regimens are becoming first-line therapy for the eradication of H pylori infections in order to remedy the current clarithromycin-resistance crisis.
Bismuth quadruple therapy provides eradication rates similar to those of clarithromycin-based therapies.1,2,9 This first-line regimen is recommended in areas of high resistance, in cases of prior macrolide exposure, and in cases where clarithromycin-based therapy has failed. The drawback of this regimen is the high pill burden; however, adherence and tolerability are similar to those for clarithromycin-based therapies. The FDA has approved a combination product (Pylera) that contains bismuth subcitrate, tetracycline, and metronidazole combined with a PPI; these agents are not FDA-approved for therapy when prescribed separately.1,2,9
Nonbismuth quadruple therapy (i.e., concomitant) for 10 to 14 days (the Toronto Consensus strongly recommends 14 days) is another first-line regimen.1,2,9 This regimen may also be used as salvage therapy in patients with persistent H pylori infection when the primary or preferred therapy fails. Although no randomized, controlled North American trials have assessed concomitant therapy, the American College of Gastroenterology (ACG) recommends its use based on a meta-analysis of 19 trials including more than 2,000 patients that demonstrated high eradication rates (mean, 88%) even in areas with high clarithromycin and metronidazole resistance.1,8,14 Notably, as with clarithromycin, metronidazole-resistance rates are also alarming (>20%).1,6
Penicillin Allergy
What is the best H pylori regimen for patients with reported penicillin allergy? Literature suggests that most patients with a documented history of penicillin allergy do not have true hypersensitivity that would preclude the use of amoxicillin-containing regimens.1 Amoxicillin is a key component in several H pylori therapies because resistance rates remain relatively low. Bismuth quadruple therapy does not contain amoxicillin and may be used in truly penicillin-allergic patients. If a penicillin-allergic patient has failed to achieve eradication after one or two regimens, allergy testing should be considered to determine whether amoxicillin-containing salvage regimens may be safely used. Additionally, a meta-analysis confirmed that triple and quadruple regimens containing doxycycline are effective in eradicating H pylori and may be considered for use in patients who cannot take amoxicillin.1,15
Alternative First-Line Regimens
The ACG conditionally recommends the use of sequential, hybrid, and fluoroquinolone-based regimens as first-line treatment, whereas the Toronto Consensus recommends against their use based on insufficient evidence.1,9 At present, levofloxacin-based therapy is an option for salvage treatment, but the ACG believes that it offers the most robust first-line efficacy data based on international trials.1
The efficacy of sequential therapy depends on the geographic region.1,2,9 Meta-analyses of global data showed an eradication rate of 84%; however, sequential therapy is not superior to triple therapy (for 14 days) or bismuth- and nonbismuth-based therapies.8,14 North American studies evaluating the efficacy of sequential therapy found no difference in efficacy compared with clarithromycin-based therapy and suggested it as a viable alternative. Sequential therapy may be an effective first-line option if used for 14 days, but further studies are needed. Additionally, the sequential regimen is complex, which may increase failure rates.1,7,8,16
Hybrid therapy merges sequential and concomitant therapies and is recognized as promising by the ACG because it has shown high cure rates in international studies.1,9 Levofloxacin-based therapy is comparable to clarithromycin triple therapy, but there is insufficient guidance on fluoroquinolone-resistance rates in North America, and evidence suggests that resistance may be higher compared with clarithromycin. (Antibiotic-resistance rates of H pylori strains in the U.S. from 2009 to 2011 reported that more than 30% of strains are resistant to levofloxacin.1) Rifabutin triple regimen with a PPI and amoxicillin and high-dose dual therapy with a PPI and amoxicillin have insufficient evidence and are not recommended.1,9
Overall, the Toronto Consensus and the ACG are in agreement about the eradication of H pylori and recommend longer treatment durations (14 days), restricting clarithromycin-based therapies, and first-line use of bismuth quadruple therapy and concomitant therapy.1,9 Given the limited antibiotic regimens to treat H pylori and increased rates of antimicrobial resistance to these antibiotics, there is a need for better long-term solutions for preventing and managing this human pathogen.
Pharmacists can serve an important function in the treatment of H pylori infections by gathering a history of previous antibiotic exposure and medication allergies and being familiar with recommended first-line and alternative first-line or salvage regimens and the factors affecting empiric regimen selection, such as resistance patterns in their geographic area. Pharmacists can also educate patients on their treatment regimen, emphasizing the importance of taking medications as prescribed in increasing the likelihood of successful eradication.
Probiotics
The use of probiotics for the management of H pylori is controversial because of inconsistent evidence and because the formulations, optimal dose, timing (before, during, or after eradication), and length of therapy are not standardized.1,9 Although the ACG suggests that probiotic therapy may be promising, the Toronto Consensus recommends against adding probiotics. Some literature suggests adding probiotics like Saccharomyces boulardii and Lactobacillus species to triple regimens to increase eradication rates (absolute increase of 9% and 5%, respectively) and to decrease adverse effects, most notably diarrhea (absolute decrease of 14% and 7%, respectively).8,17,18 However, the addition of probiotics increases the cost and pill burden of an already-complex regimen.
Monitoring
Ideally, all patients would undergo testing for H pylori eradication to confirm successful treatment as well as to track rates of H pylori; however, it is not cost-effective to confirm eradication in all treatment groups. Indications for confirmatory H pylori–eradication testing include H pylori–associated ulcer, persistent dyspeptic symptoms, H pylori–associated MALT lymphoma, and resection of early gastric cancer. Confirmatory tests should be conducted at 4 to 8 weeks following therapy.5 The UBT is the best option for confirmation of H pylori eradication; the SAT is an alternative.2,5 Serology can detect past H pylori infections and should not be used to monitor effectiveness.2,9
Conclusion
H pylori is a globally prevalent, high-risk pathogen. Recommended testing for H pylori has been expanded, and all patients who test positive should be treated. The UBT is best for detection and eradication. Antibiotics and bismuth should be held for at least 4 weeks and PPIs should be held for at least 2 weeks prior to all H pylori diagnostic tests except serology. Successful eradication of H pylori is based on bacterial and host factors. Triple therapy with clarithromycin was historically first-line treatment; however, increasing clarithromycin resistance necessitates additional first-line therapies. To select the most efficacious empiric regimen, patients should be asked about prior macrolide use and medication allergies. Evidence regarding probiotics for H pylori treatment is inconsistent. Pharmacists should be familiar with the treatment regimens for H pylori and educate patients on the importance of adherence. Testing for eradication is recommended in specific patient groups 4 to 8 weeks following completion of treatment.
REFERENCES
1. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239.
2. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30.
3. World Gastroenterology Organisation Global Guideline: Helicobacter pylori in developing countries. J Clin Gastroenterol. 2011;45:383-388.
4. Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20:299-304.
5. Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825.
6. Alba C, Blanco A, Alarcón T. Antibiotic resistance in Helicobacter pylori. Curr Opin Infect Dis. 2017;30:489-497.
7. De Francesco V, Bellesia A, Ridola L, et al. First-line therapies for Helicobacter pylori eradication: a critical reappraisal of updated guidelines. Ann Gastroenterol. 2017;30:373-379.
8. Fashner J, Gitu AC. Diagnosis and treatment of peptic ulcer disease and H. pylori infection. Am Fam Physician. 2015;91:236-242.
9. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori Infection in adults. Gastroenterology. 2016;151:51-69.e14.
10. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153.
11. Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori–infected persons, United States. Emerg Infect Dis. 2004;10:1088-1094.
12. McNulty CA, Lasseter G, Shaw I, et al. Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment Pharmacol Ther. 2012;35:1221-1230.
13. Abadi ATB. Resistance to clarithromycin and gastroenterologist’s persistence roles in nomination for Helicobacter pylori as high priority pathogen by World Health Organization. World J Gastroenterol. 2017;23:6379-6384.
14. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23-34.
15. Niv Y. Doxycycline in eradication therapy of Helicobacter pylori—a systematic review and meta-analysis. Digestion. 2016;93:167-173.
16. Georgopoulos SD, Xirouchakis E, Martinez-Gonzales B, et al. Randomized clinical trial comparing ten day concomitant and sequential therapies for Helicobacter pylori eradication in a high clarithromycin resistance area. Eur J Intern Med. 2016;32:84-90.
17. Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother. 2011;45:960-966.
18. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
19. Moayyedi PM, Lacy BE, Andrews CN, et al. ACG and CAG Clinical Guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988-1013.
20. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328.
To comment on this article, contact rdavidson@uspharmacist.com.





