ABSTRACT: When hospitalization is required to manage an accidental injury or complications of a worsening chronic disease, the health team is challenged to not only focus on the presenting diagnoses, but also to minimize additional risks to the patient while under inpatient care. In addition to monitoring progress to achieve clinical goals, providers must also be vigilant in preventing additional risks such as adverse drug reactions and preventable medication errors. An additional monitoring focus that may be less commonly considered, but equally important, is the potential emergence of psychiatric symptoms in patients without a history of mental illness. Delirium, exacerbations of perceptual symptoms in dementia, and psychosis are chief among these considerations because of the significant stress they can cause the patient and caregivers.
Among the major challenges of the current healthcare system is providing effective community-based care for dependent older adults and other frail, community-dwelling individuals who have been diagnosed with multiple chronic conditions. Studies have demonstrated that the provision of integrated care at the patient’s home not only reduces unnecessary hospitalizations and emergency-room visits, but also decreases earlier mortality in these vulnerable community-dwelling individuals.1 Despite systemwide efforts to reduce hospitalizations by more effectively managing chronic disease in the community, many individuals will still require inpatient intervention; therefore, the focus must be on decreasing length of hospital stay and reducing or avoiding unintended complications during hospitalization, regardless of the length of stay.
Given the prominent administrative and quality-assurance focus on key areas identified as either National Patient Safety Goals or Joint Commission Standards, the more common but equally complicated patient-care concerns often are overlooked. One such care concern is the emergence of unexpected psychosis during hospital admission in a patient not previously diagnosed with mental illness or the exacerbation of psychiatric symptoms in patients with a known mental-health disorder.2,3
Delirium and Associated Psychotic Symptoms
Current healthcare advances have led to a decrease in mortality associated with acute critical-care illness; however, research has demonstrated that patients who experience and recover from critical illness frequently do not return to their baseline cognitive status. As many as 60% of these patients continue to be challenged with significantly increased impairment due to this acquired dementia (long-term cognitive impairment after critical illness) months and even years after a stay in the ICU.4
Common in critically ill patients, delirium is a condition of acute brain dysfunction that manifests as a cluster of psychiatric symptoms in a patient without known psychiatric illness. Delirium, or acute confusion, is associated with poorer outcomes, prolonged hospitalization, and cognitive impairment.5 This serious health condition is more common in patients with advanced age, but it also presents in younger patients with severe and acute medical illness. Up to 80% of patients admitted to palliative care and ICUs are reported to experience delirium. Delirium, often emerging with a highly variable presentation, has symptoms including agitation, altered awareness, and decreased responsiveness. Some patients may also exhibit perceptual disturbances, which include delusions and hallucinations.5
Postoperative delirium (POD) is defined as a temporary state of confusion and disorientation that occurs acutely, often immediately after surgery, but emergence may be delayed and occur several days postoperatively. Because patients may emerge from surgery in a seemingly normal state, identification and understanding of at-risk patients becomes even more important when establishing protocols for surveillance and treatment readiness to address potential psychosis and other symptoms associated with delirium. Because POD, otherwise known as ICU psychosis or ICU syndrome, is a common postsurgical complication in older adults that often may lead to other undesirable sequelae such as long-term cognitive impairment, it is important to understand that the best management strategy is prevention through recognition of those patients who are at high risk.6,7
One such high-risk group includes patients requiring mechanical ventilation in the ICU. Studies have reported that up to 80% of patients requiring mechanical ventilation in the ICU experience delirium, a condition that is predictively related to potential for cognitive decline in older adults. Several other studies have supported advanced age as an additional high-risk factor and have reported the development of delirium even in older inpatients not admitted to the ICU (without critical illness) who experienced long-term cognitive impairment after inpatient treatment. Although the manifestation of ICU delirium has been recognized to increase the risk of mortality, the duration of delirium has been reported to be an independent risk factor for the likelihood of long-term cognitive impairment that can last up to a year after recovery from critical illness.4
It has been estimated that more than 30% of delirium cases are preventable and that up to 60% of cases may be unrecognized. Although prevention is the optimal strategy in addressing delirium, those cases that occur require accurate diagnosis and quick intervention (see TABLE 1).5 Nonpharmacologic intervention is the first line of treatment (see TABLE 2)5; however, according to current guidelines from the National Institute for Health and Care Excellence, short-term use of haloperidol or olanzapine is considered appropriate, with haloperidol being associated with a greater risk of extrapyramidal symptoms and olanzapine with a greater degree of sedation.8

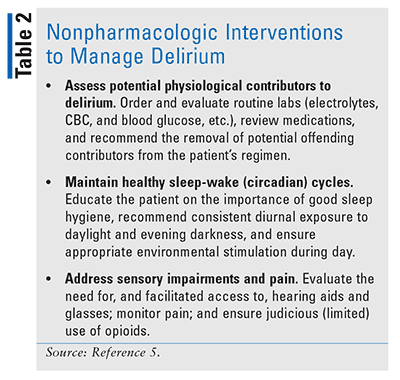
Pharmacologic Interventions for Delirium
There is currently no irrefutable evidence supporting the use of pharmacotherapy to prevent delirium; however, the use of antipsychotics, cholinesterase inhibitors, and psychostimulants has been explored for this purpose.9 The avoidance of potentially problematic pharmacotherapy is the most significant intervention in preventing delirium. Examples include the use of antipsychotics in populations with a risk of increased mortality, such as patients with dementia-related psychosis, and benzodiazepines in patients particularly vulnerable to the sedating effects of these agents, in the absence of severe agitation and psychosis. In the ICU setting, dexmedetomidine used in lieu of classic sedatives may prevent and shorten the duration of delirium.10
Dementia-Related Psychosis
Dementia is a progressively degenerative neurologic condition associated with several different diseases. Alzheimer’s disease (AD) accounts for up to 80% of dementia cases, outnumbering the cases of dementia caused by Lewy bodies or vascular injury.11 Cur-rent therapeutic interventions for dementia include the use of pharmacotherapy to slow disease progression, since there is currently no cure for AD, and to control the behavioral symptoms that cause the patient stress and carry a significant caregiver burden.12 Both the behavioral and associated psychiatric symptoms, including but not limited to psychosis, may overwhelm caregivers and can further reduce the quality of life and independence of the patient. It is often these secondary symptoms associated with the progression of dementia that lead to earlier inpatient hospitalization and institutionalization; therefore, they should be an additional clinical priority.12
Frail elderly patients, including those with dementia, often experience cognitive decline compared with their own personal baseline when recovering from acute illness.13 For this reason, it is especially important to be mindful that a patient with seemingly stable mental status, even with mild-stage dementia prior to admission, can experience an emergence of psychosis and exacerbation of behavioral symptoms during hospitalization. Changes in environment, including alterations in the sleep-wake cycle and lack of a diurnal schedule of day-to-night light exposure that the individual is accustomed to at home, contribute to the disrupted circadian cycle. Similarly, behaviors that worsen at night have been termed sundowning because the worsening occurs when the sun sets in the evening.14 Additionally, the stress experienced by the patient with dementia upon the introduction of unfamiliar people intervening during a medical crisis is compounded by healthcare staff in the emergency room, who often have limited training in treating patients with dementia.15
Dementia-related psychosis is not uncommon, when considering all the other disease-induced causes of dementia that have been associated with psychosis, including Parkinson’s disease, HIV, head trauma, and Huntington disease. Symptoms of dementia can mimic those of other conditions; therefore, when evaluating psychosis that is thought to be attributed to medical illness or potential neurologic disease, clinicians should rule out other causes, such as untreated infection, endocrine disorders (hypothyroidism, hypercalcemia, hypoglycemia), vitamin deficiency (thiamin, niacin), immune disorders (lupus), and other conditions in order to avoid a delay in appropriate treatment.16
Treatment for psychosis in patients with dementia, whether they are hospitalized or in the community, is challenging. The FDA has not approved any medication specifically for treatment, and it has issued a classwide black box warning (BBW) advising against the use of antipsychotic agents for the treatment of dementia-related psychosis.17 This warning details the risk of increased mortality when antipsychotic agents are used in these patients. The studies that support the BBW revealed an increased mortality of 1.6 to 1.7 times for patients treated with antipsychotic agents compared with those given placebo. Most patients experienced death caused by cardiovascular complications (such as sudden death or heart failure) or infection (pneumonia). The FDA advises that although the studies are limited by the inability to determine whether this increased risk was due to patient-specific characteristics or the antipsychotic agents alone, or a whether there was a confounding effect of both, these agents are not approved for the treatment of patients with dementia-related psychosis.17-19
Given the lack of FDA-approved medications, along with the limitations imposed by the Omnibus Budget Reconciliation Act of 1987 and the American Geriatric Society’s Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, prescribers may find themselves without adequate alternatives for patients who require relief from psychotic symptoms.20,21 These restrictions have encouraged many clinicians to explore off-label prescribing, including the use of polypharmacy, in an effort to reduce behavioral symptoms. The use of polypharmacy in older adults greatly increases the risk of drug-drug interactions and adverse drug reactions. Acute symptoms such as increased confusion, psychosis, and psychomotor retardation may be signs of delirium, which is a common co-occurring condition in patients with dementia.11
By 2050, the number of Americans aged 65 years or older is expected to be 88.5 million, more than double that in 2010. Because the life expectancy of the overall population is increasing, so too is the number of people diagnosed with dementia because advanced age is one of the strongest risk factors.22
Parkinson’s Disease Psychosis
Patients with Parkinson’s disease (PD) are best recognized by the hallmark motor symptoms of the disease; however, the nonmotor symptoms, including hallucinations and delusions, may be left undertreated or entirely unaddressed if the patient or caregivers do not report their occurrence. Because providers often focus preferentially on the motor symptoms, they may be less aware of the nonmotor manifestations and may fail to inquire further of the patient and caregivers.23 The risk of psychiatric symptoms increases when a patient with PD also has dementia, with roughly 75% of patients with concurrent PD developing psychosis and other related psychiatric symptoms.23 There is a highly variable individualized spectrum of PD psychosis (PDP) symptoms, which range from mild visual distortions to significant visual hallucinations, in which individuals report seeing images that are not real. Patients may also experience delusions of fixed false beliefs that become the individual’s reality.24
Although the normal course of PDP is a gradual worsening with advancing illness severity, polypharmacy can be a key factor in exacerbating these psychiatric symptoms. For this reason, a patient presenting with acute medical illness who is managed with an increased burden of medications, including anticholinergic agents for allergies, incontinence, or sleep, may experience a newly emergent psychosis or a worsening of existing psychotic symptoms.25,26 Additionally, dopaminergic medications used therapeutically to lessen the PD motor symptoms can exacerbate the psychiatric PDP symptoms. For this reason, upon emergence of PDP in a medically ill patient, deprescribing strategies should be employed, with the drugs with higher risk (and lowest benefit) eliminated initially and additional discontinuations implemented until a balance is established between control of the PD symptoms and control of drug-induced PDP. 25,26
Pharmacologic Interventions for PDP
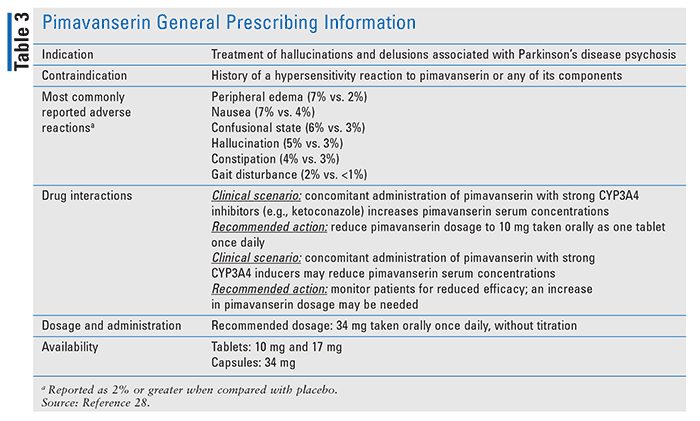
Although there are additional nonpharmacologic approaches that can be implemented to mitigate environmental causes, pharmacologic interventions may still be necessary if deprescribing efforts do not establish the planned therapeutic balance. Prior to the launch of pimavanserin (Nuplazid) in April 2016, there were no antipsychotic agents available to improve symptoms in these patients.27 Because of the complexity of the competing actions of dopamine receptors, with antagonism being a primary mechanism of action for antipsychotic agents, and the need for dopamine agonists to suppress PD motor symptoms caused by the dopamine deficit of PD, patients with PDP were in a medication-management gridlock. The administration of antipsychotics effectively reduced the positive symptoms (including psychosis); however, they also worsened the movement disorders that are the hallmark of PD. Pimavanserin is a novel antipsychotic agent that is a serotonin inverse agonist with low binding affinity to dopamine receptors (see TABLE 3).26,28 As described earlier, the FDA-mandated BBW advises against the use of antipsychotic agents for patients with dementia-related psychosis, and therefore the use of pimavanserin is not approved for treating patients with dementia-related psychosis unrelated to hallucinations and delusions associated with PDP.17,18
The Role of the Pharmacist
The pharmacist, whether part of the inpatient treatment team or collaborating medication-management partner in the community pharmacy, plays a vital role in ensuring that patients who experience unexpected psychiatric symptoms during hospitalization are evaluated at the time of occurrence and are educated about the different causes of the psychiatric symptoms experienced during their hospitalization upon discharge. On the inpatient side, pharmacists can assist in the assessment of contributing causes, especially when evaluating the potential to deprescribe potentially unnecessary medications found in a high-burden drug regimen. In all patient-care settings, the pharmacist serves a key role as educator for the patients and caregivers and as a liaison between psychiatry and medicine to assist in coordinating safe and effective use.
Furthermore, because older, chronically medically ill patients are likely taking multiple medications prescribed by different care providers to address a variety of medical and neurologic comorbidities, the potential for drug-drug interactions and adverse drug events is significant. Pharmacists can also provide information relative to care between office visits and when medication adjustments are implemented to offer effective strategies to manage symptoms as they emerge. Finally, the pharmacist may contribute expertise in evaluating the potential drug-induced causes of psychiatric exacerbation, including, but not limited to, mania and/or psychosis due to illicit-substance use.
REFERENCES
1. Di Pollina, L, Guessous, I, Petoud, V, et al. Integrated care at home reduces unnecessary hospitalizations of community-dwelling frail older adults: a prospective controlled trial. BMC Geriatrics. 2017;17(1):53.
2. The Joint Commission Antimicrobial Stewardship Standard 2018; www.jointcommission.org/topics/hai_antimicrobial_stewardship.aspx Accessed August 28, 2018.
3. Agency for Healthcare Research and Quality When Psychiatric Symptoms are Not (AHRQ) https://psnet.ahrq.gov/webmm/case/5/when-psychiatric-symptoms-are-not
Accessed August 28, 2018.
4. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine. 2010;38(7):1513.
5. Oldham MA, Flanagan NM, Khan A, et al. Responding to ten common delirium misconceptions with best evidence: an educational review for clinicians. J Neuropsychiatry and Clinical Neurosciences. 2017;30(1):51-57.
6. Bourne R. Delirium in the intensive care unit. In Case Studies in Adult Intensive Care Medicine. United Kingdom: Cambridge University Press; 2017:296-297.
7. Johnson MP. Postoperative delirium as a complication of maxillofacial surgery. In Perioperative Assessment of the Maxillofacial Surgery Patient. 2018;625-634. Springer, Cham.
8. Young J, Murthy L, Westby M , et al. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. www.nice.org.uk/guidance/cg103/chapter/1-Guidance#treating-delirium. Accessed August 28, 2018.
9. Kluger C, Shah P, Maiti S, et al. Therapeutic advances in the prevention and treatment of delirium in the hospital setting. American Journal of Therapeutics. 2018;25(1):e3-e14.
10. Wang DS, Kaneshwaran K, Lei G, et al. Dexmedetomidine prevents excessive γ-aminobutyric acid type A receptor function after anesthesia. Anesthesiology. 2018.
11. The Alzheimer’s Association. What is Alzheimers? www.alz.org/alzheimers-dementia/what-is-alzheimers. Accessed August 28, 2018.
12. Demler TL. The inpatient with dementia. US Pharm. 2014;39(11):HS18-HS25. www.uspharmacist.com/article/the-inpatient-with-dementia. Accessed August 8, 2018.
13. Moore G, Hartley P, Romero‐Ortuno R. Health and social factors associated with a delayed discharge amongst inpatients in acute geriatric wards: a retrospective observational study. Geriatrics & Gerontology International. 2018;18(4):530-537.
14. Alzheimers Association. Sleep issues and sundowning. www.alz.org/help-support/caregiving/stages-behaviors/sleep-issues-sundowning. Accessed August 29, 2018.
15. NIAD National Institute of Aging. AD in the hospital. www.nia.nih.gov/health/going-hospital-tips-dementia-caregivers. Accessed August 29, 2018.
16. Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. The Lancet Neurology. 2015;14(8):823-832.
17. The U.S. Food and Drug Administration. Black box warning and antipsychotic agents (Zyprexa). www.accessdata.fda.gov/drugsatfda_docs/nda/2008/020592orig1s049.pdf accessed. August 8, 2018.
18. Demler, TL. Generic drugs and black box warnings: critical information for patient safety. US Pharm. 2017;(6)(Generic Drugs suppl):6-12. www.uspharmacist.com/article/generic-drugs-and-black-box-warnings-critical-information-for-patient-safety. Accessed August 8, 2018.
19. Centers for Medicare & Medicaid Services (CMS) Atypical Antipsychotic Medications: Use in Adults. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/atyp-antipsych-adult-factsheet11-14.pdf. Accessed August 29, 2018.
20. Omnibus Budget Reconciliation Act of 1987 (OBRA). Subtitle C, nursing home reform. PL100-203. Washington, DC: National Coalition for Nursing Home Reform; 1987.
21. AGS Beers criteria for potentially inappropriate medication use in older adults. American Geriatric Society; 2012. www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed August 28, 2018.
22. Vincent GK, Velkoff, VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. Washington, DC: U.S. Census Bureau; May 2010. www.census.gov/prod/2010pubs/p25-1138.pdf Accessed August 28, 2018.
23. Hermanowicz N, Edwards K. Parkinson’s disease psychosis: symptoms, management, and economic burden. The American journal of managed care. 2015;21(10 suppl):s199-s206.
24. Martinez-Martin P, Rodriguez-Blazquez C, Forjaz M J, et al. Neuropsychiatric symptoms and caregiver’s burden in Parkinson’s disease. Parkinsonism & Related Disorders. 2015;21(6):629-634.
25. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
26. Goldman JG, Vaughan CL, Goetz CG. An update expert opinion on management and research strategies in Parkinson’s disease psychosis. Expert Opinion on Pharmacotherapy. 2011;12(13):2009-2024.
27. Hacksell U, Burstein ES, McFarland K, et al. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochemical Research. 2014;39(10):2008-2017.
28. Nuplazid (pimavanserin) package insert. San Diego, CA: Acadia; June 2018.
To comment on this article, contact rdavidson@uspharmacist.com.






