US Pharm. 2017;42(2)(Specialty&Oncology suppl):7-11.
ABSTRACT: Cases of soft-tissue sarcoma (STS), which accounts for about 1% of adult cancers and 15% of pediatric cancers, are generally underreported because they often are asymptomatic and lymph node development is infrequent. It was estimated that there would be 12,310 cases of newly diagnosed STS and approximately 4,990 deaths in the United States in 2016. STS is relatively resistant to chemotherapy, and surgery is the primary treatment in most cases. There are no standard chemotherapy regimens; selection of agent and treatment outcome are determined by the site and type of STS (more than 50 subtypes exist), and clinical trials serve as first-line therapy for metastatic STS. Since 2015, the FDA has approved three agents for the treatment of STS: olaratumab, trabectedin, and eribulin mesylate.
Sarcomas are solid tumors of which there are many different subtypes, each with its own clinical and pathologic features. Sarcomas may be divided into two broad categories: bone sarcomas and sarcomas of soft tissue (including fat, muscle, nerve and nerve sheath, blood vessels, and other connective tissues). Sarcomas are relatively rare in adults (about 1% of all adult cancers) and account for 15% of pediatric cancers. Adult sarcomas are more common in soft tissue than in bone.1 It was estimated that 12,310 cases of soft-tissue sarcoma (STS) would be newly diagnosed in the United States in 2016 and that 4,990 STS-related deaths would occur.2 A large proportion of cases of gastrointestinal stromal tumors (GISTs) may not have been reported prior to 2001; as a result, the incidence of STS is underestimated. In the U.S., approximately 5,000 new cases of GISTs occur annually.3
STS Subtypes
There are more than 50 different subtypes of STS. Common subtypes include leiomyosarcoma (23.9%), undifferentiated pleomorphic sarcoma (previously called malignant fibrous histiocytoma; 17.1%), GISTs, sarcoma not otherwise specified (12.8%), liposarcoma (11.5%), dermatofibrosarcoma (10.5%), rhabdomyosarcoma (4.6%), and angiosarcoma (4.1%).4 The various subtypes of STS present in different locations of the body. Most STSs occur in the lower extremities (30%), followed by the abdominal viscera (21%), abdominal retroperitoneum (17%), upper extremities (15%), head and neck (13%), and trunk (10%).
Disease Progression
STS is usually asymptomatic, and regional lymph node development is infrequent.4 Metastasis typically develops in later stages of the disease, correlating with the size and histologic grade of the tumor. STS usually metastasizes to the lungs, or to the liver if the STS is retroperitoneal.
Treatment
The anatomical site of the primary disease is an important variable that influences treatment and outcome. Surgery is the standard primary treatment for most cases of STS, especially in earlier disease stages. A variety of surgical methods are employed, such as radical excision (commonly using a 5-cm margin), limb-sparing surgery, compartmental excision, and amputation, but limb-sparing surgery is possible in most patients. Radiation may be given preoperatively, postoperatively, or as primary treatment. Brachytherapy, intraoperative radiation therapy, and intensity-modulated radiation therapy have led to improved outcomes in STS.5
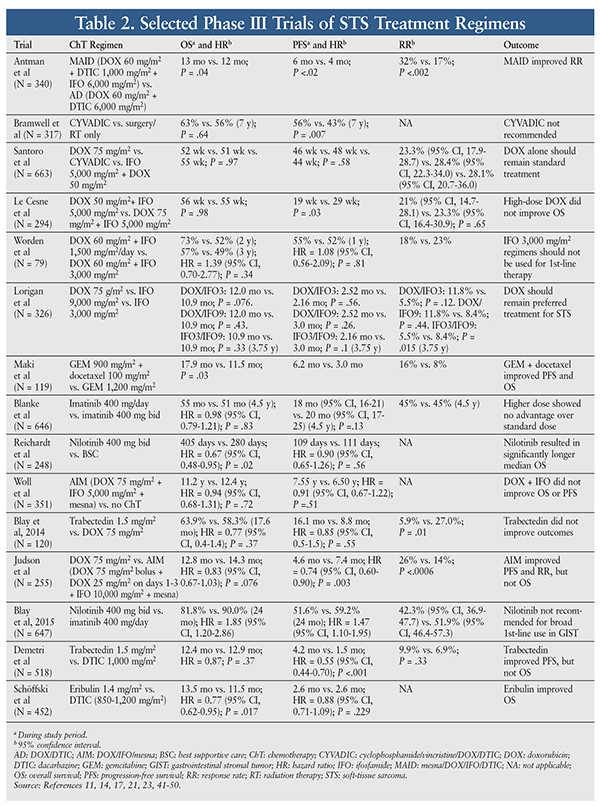
STS is relatively resistant to chemotherapy, whose efficacy against STS is variable and largely unsatisfactory. In general—with the exception of GISTs—the choice of agent and level of response depend on the type of STS.5 Because of insufficient data, there are no specific preferred treatment options for metastatic STS; therefore, a clinical trial should be considered. However, a variety of chemotherapy agents, alone or in combination, may be used for metastatic, unresectable, or advanced STS. The pharmacology, dosing, and adverse events (AEs) of chemotherapy agents used for common STS subtypes are highlighted in TABLE 1. Outcomes of selected clinical trials involving these agents are summarized in TABLE 2.
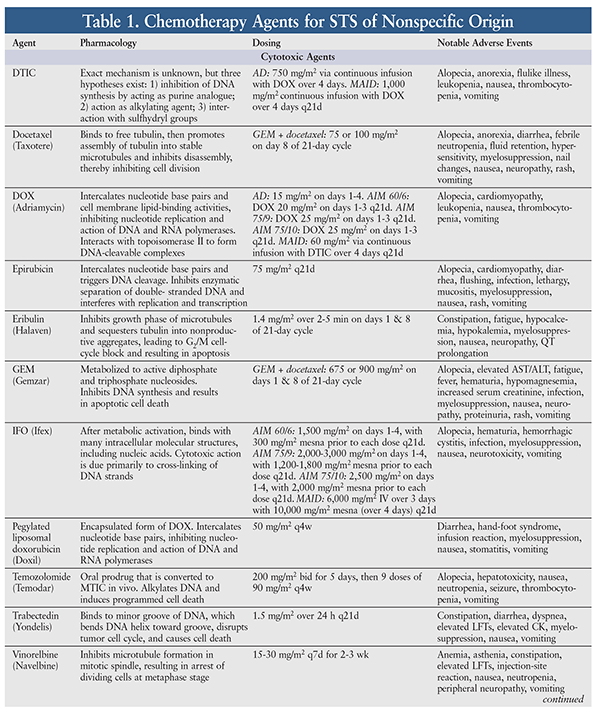
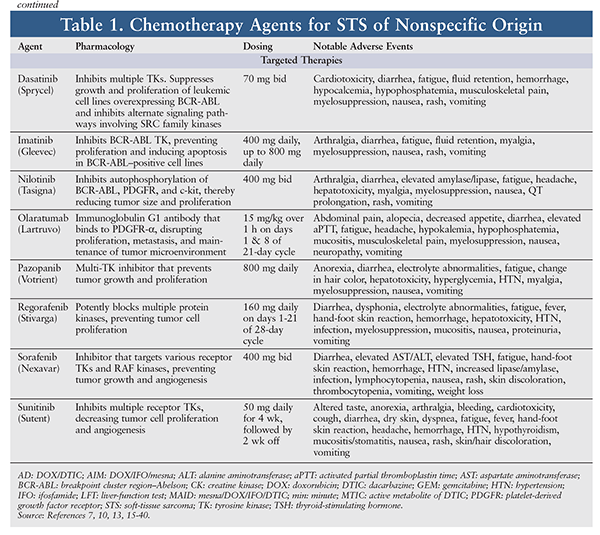
Pharmacologic therapy is better defined for GISTs than for other types of STS.5 For resectable GISTs with negative margins, preoperative imatinib 400 mg daily may be beneficial as primary treatment. If the GIST possesses the KIT exon 9 mutation, the dose may be escalated to 400 mg twice daily as tolerated. Treatment should continue until there is no further disease improvement on two consecutive CT scans. For unresectable, metastatic, or recurrent GISTs, imatinib may be initiated at 400 mg daily; after 3 months, if the tumor is unresectable, imatinib should be continued. Upon further progression, the dose may be escalated to 400 mg twice daily. If the disease is no longer controlled on imatinib, sunitinib may be initiated until further progression. If the disease progresses on sunitinib, regorafenib may be used. If treatment with imatinib, sunitinib, or regorafenib fails, a previously effective tyrosine kinase (TK) inhibitor may be reintroduced. Treatment may continue for as long as clinical benefit is evident.5
New Chemotherapy Agents
Since 2015, three new chemotherapy agents have been approved for the treatment of STS: olaratumab (Lartruvo), trabectedin (Yondelis), and eribulin mesylate (Halaven).
Olaratumab: Olaratumab was granted accelerated approval in October 2016 for the treatment of STS with a non-Kaposi’s histologic subtype (i.e., leiomyosarcoma [LMS], liposarcoma, and synovial sarcomas) that is susceptible to anthracyclines and is not amenable to radiation therapy or surgery.6 Olaratumab is a human immunoglobulin G1 monoclonal antibody that binds to platelet-derived growth factor receptor-alpha (PDGFR-alpha), which is a TK expressed on mesenchymal cells. PDGFR-alpha, which has also been found on other stromal cells and tumor cells, such as sarcomas, contributes to tumor cell proliferation and metastasis.7
Approval of olaratumab was based on a phase II, multicenter, open-label, randomized trial in 133 patients with metastatic STS not curable with surgery or radiation therapy and sarcoma histology responsive to an anthracycline-containing regimen.8 Patients were randomized to receive either olaratumab 15 mg/kg IV on days 1 and 8 plus doxorubicin 75 mg/m² or doxorubicin monotherapy on day 1 of a 21-day cycle for up to eight cycles. Patients’ median age was 58 years, more than 80% were white, and about two-thirds had had no prior chemotherapy. Thirty-eight percent of the combination group had LMS, and 61% had more than 25 other STS histologies. Patients in the combination group experienced an improvement in overall survival (OS), with a median of 26.5 months (95% CI, 20.9-31.7) versus 14.7 months (95% CI, 9.2-17.1) in the doxorubicin-only group (hazard ratio [HR] = 0.46 [95% CI, 0.30-0.71]). Median progression-free survival (PFS) was 6.6 months (95% CI, 4.1-8.3) in the combination group versus 4.1 months (95% CI, 2.8-5.4) in the doxorubicin-only group (HR = 0.672 [95% CI, 0.442-1.021]; P = .0615). Overall response rate was 18.2% (95% CI, 9.8-29.6) in the combination group versus 11.9% (95% CI, 5.3-22.2) in the doxorubicin-only group.8
The incidence of treatment-related AEs was similar between groups; however, the combination group had more AEs of grade 3 or higher.8 Grade 3 fatigue and neutropenia occurred at rates of 9% and 19%, respectively, in the combination group compared with 3% and 8%, respectively, in the doxorubicin-only group. Infusion reactions occurred in 13% (2% were grade 4 or higher) of the combination group compared with 0% of doxorubicin-only patients. The prevalence of cardiac dysfunction was similar between groups, with a slightly higher incidence in the combination group (23% vs. 17%). The combination group had higher rates of mucositis (53% vs. 35%), musculoskeletal pain (64% vs. 25%), alopecia (52% vs. 40%), constipation (34% vs. 32%), decreased appetite (31% vs. 20%), nausea (73% vs. 52%), vomiting (45% vs. 18%), and diarrhea (34% vs. 23%).8
Olaratumab is dosed at 15 mg/kg and administered via IV infusion over 60 minutes on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity occurs.7 It should be administered with doxorubicin for the first eight cycles. Patients should also receive diphenhydramine 25 to 50 mg IV and dexamethasone 10 to 20 mg IV prior to olaratumab on day 1 of cycle 1. No dosage adjustments are necessary in renal or hepatic impairment; however, treatment should be withheld in the setting of a grade 4 infusion reaction or neutropenia lasting longer than 1 week.7
Trabectedin: This agent was approved in October 2015 for the treatment of unresectable or metastatic LMS and liposarcoma in patients who have previously received anthracycline-containing therapies.9 Trabectedin, an alkylating agent, binds to the minor groove of DNA, which bends the DNA helix toward the groove, disrupts the tumor cell cycle, and causes cell death.10
Approval of trabectedin was based on a phase III, multicenter, open-label, randomized trial in 518 patients with advanced or metastatic liposarcoma and LMS randomized in a 2-to-1 fashion to receive either trabectedin at a starting dose of 1.5 mg/m2 as a 24-hour IV infusion or dacarbazine at a starting dose of 1,000 mg/m2 as a 20- to 120-minute IV infusion. The trabectedin group experienced an improvement in median PFS of 4.2 months, compared with 1.5 months in the dacarbazine group (HR = 0.55; 95% CI, 0.44-0.70; P <.001). An interim analysis for the primary endpoint of OS showed a nonsignificant difference between groups: 12.4 months in the trabectedin arm versus 12.9 months in the dacarbazine group (HR = 0.87; P = .37).11
AEs in this study were consistent with those in previous studies of the safety and toxicity of trabectedin.11 AEs, most of which were not severe, included nausea (73% vs. 49%), fatigue (67% vs. 51%), vomiting (44% vs. 21%), constipation (36% vs. 28%), decreased appetite (34% vs. 20%), diarrhea (34% vs. 23%), dyspnea (25% vs. 19%), and peripheral edema (24% vs. 14%). Severe AEs included thrombocytopenia (17% vs. 18%), neutropenia (37% vs. 21%), alanine aminotransferase elevation (26% vs. 1%), aspartate aminotransferase elevation (13% vs. 0%), and rhabdomyolysis (1.2% vs. 0%).11 Although it did not improve OS, trabectedin is still a treatment option for patients who have experienced failure of previous chemotherapy.
Trabectedin is dosed at 1.5 mg/m2 and administered via a 24-hour central line infusion every 3 weeks.10 Patients should also receive premedication with dexamethasone 20 mg IV 30 minutes prior to trabectedin administration. Dosage adjustment is required for hepatic impairment. Baseline hepatic function and left ventricular ejection fraction should be obtained prior to initiating treatment.10
Eribulin: Eribulin mesylate was approved in January 2016 for the treatment of unresectable or metastatic liposarcoma in patients who have previously received anthracycline-containing therapies.12 Originally approved for metastatic breast cancer in 2010, this agent inhibits the growth phase of microtubules and sequesters tubulin into nonproductive aggregates, leading to G2/M cell-cycle block and apoptotic cell death.13
Approval was based on a phase III, multicenter, open-label, randomized trial in 452 patients with intermediate-grade or high-grade advanced liposarcoma or LMS who had received at least two previous systemic regimens for advanced disease (including one containing an anthracycline).14 The trial compared eribulin 1.4 mg/m² IV over 2 to 5 minutes on days 1 and 8 of a 21-day cycle with dacarbazine 850 mg/m², 1,000 mg/m², or 1,200 mg/m² IV over 15 to 60 minutes on day 1 of a 21-day cycle. The starting dose of dacarbazine was determined by the patient’s clinical status and institutional preference. Median OS was longer in the eribulin group than in the dacarbazine group, at 13.5 months (95% CI, 10.9-15.6) and 11.5 months (95% CI, 9.6-13.0), respectively (HR = 0.77; 95% CI, 0.62-0.95; P = .0169). In the advanced liposarcoma subgroup, eribulin patients had an advantage in OS of 15.6 months (95% CI, 10.2-18.6) versus 8.4 months (95% CI, 5.2-10.1) in dacarbazine patients (HR = 0.51; P = .0169). In the leiomyosarcoma subgroup, OS was 12.7 months (95% CI, 9.8-14.8) in eribulin patients and 13.0 months (95% CI, 11.3 15.1) in dacarbazine patients, respectively. Although this was an intriguing finding, the study was not powered to assess subgroup analyses. The PFS was 2.6 months in both the eribulin and dacarbazine groups (HR = 0.88; 95% CI, 0.71-1.09; P = .229).14
Eribulin is dosed at 1.4 mg/m2 and administered IV over 2 to 5 minutes on days 1 and 8 of a 21-day cycle.13 Dosage adjustments are required for hepatic and renal impairment, and the drug should be withheld if the absolute neutrophil count is <1,000/mm3 or platelets are <75,000/mm3.13
Pharmacist’s Role
Pharmacists can have an impact on the therapies of their patients with STS. They can assist in determining the dosing of chemotherapy agents and in creating optimal strategies to manage and prevent AEs associated with therapy. Pharmacists should monitor laboratory values for efficacy and safety, as well as recommend any potential dose adjustments, if needed. As the use of oral chemotherapy agents that patients can self-administer at home becomes more prevalent, pharmacists can play a vital role in educating patients on adherence, proper administration, and self-monitoring.
REFERENCES
1. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
2. Siegel R, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
3. D’Amato G, Steinert DM, McAuliffe JC, Trent JC. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control. 2005;12:44-56.
4. Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914-1926.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma. Version 2.2016. www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed October 14, 2016.
6. FDA. FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Accessed January 4, 2017.
7. Lartruvo (olaratumab) package insert. Indianapolis, IN: Eli Lilly and Co; October 2016.
8. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomized phase 2 trial. Lancet. 2016;388:488-497.
9. FDA. FDA approves new therapy for certain types of advanced soft tissue sarcoma. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468832.htm. Accessed January 4, 2017.
10. Yondelis (trabectedin) package insert. Horsham, PA: Janssen Products, LP; July 2016.
11. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786-793.
12. FDA. FDA approves first drug to show survival benefit in liposarcoma. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm483714.htm. Accessed January 4, 2017.
13. Halaven (eribulin mesylate) package insert. Woodcliff Lake, NJ: Eisai Inc; January 2016.
14. Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629-1637.
15. Dacarbazine package insert. Lake Forest, IL: Hospira, Inc; January 2016.
16. Adkins KE, Solimando DA Jr, Waddell JA. Doxorubicin and dacarbazine (AD) regimen for soft tissue sarcomas. Hosp Pharm. 2015;50:194-198.
17. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276-1285.
18. Taxotere (docetaxel) package insert. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; December 2015.
19. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824-2831.
20. Adriamycin (doxorubicin) package insert. New York, NY: Pfizer Inc; January 2014.
21. Worden FP, Taylor JM, Biermann JS, et al. Randomized phase II evaluation of 6 g/m2 of ifosfamide plus doxorubicin and granulocyte colony-stimulating factor (G-CSF) compared with 12 g/m2 of ifosfamide plus doxorubicin and G-CSF in the treatment of poor-prognosis soft tissue sarcoma. J Clin Oncol. 2005;23:105-112.
22. Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667-1672.
23. Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415-423.
24. Epirubicin hydrochloride package insert. Irvine, CA: Teva Parenteral Medicines, Inc; 2010.
25. Nielsen O, Dombernowsky P, Mouridsen H, et al. High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer. 1998;78:1634-1639.
26. Gemzar (gemcitabine) package insert. Indianapolis, IN: Lilly USA, LLC; June 2014.
27. Ifex (ifosfamide) package insert. Deerfield, IL: Baxter Health Corp; November 2008.
28. Doxil (doxorubicin hydrochloride liposome) package insert. Horsham, PA: Janssen Products, LP; June 2016.
29. Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37:870-877.
30. Temodar (temozolomide) package insert. Whitehouse Station, NJ: Merck & Co, Inc; September 2015.
31. Talbot SM, Keohan ML, Hesdorffer M, et al. A phase II trial of temozolomide in patients with unresectable or metastatic soft tissue sarcoma. Cancer. 2003;98:1942-1946.
32. Vinorelbine package insert. Schaumberg, IL: Sagent Pharmaceuticals; May 2014.
33. Anderson SE, Keohan ML, D’Adamo DR, Maki RG. A retrospective analysis of vinorelbine chemotherapy for patients with previously treated soft-tissue sarcomas. Sarcoma. 2006;2006:15947.
34. Sprycel (dasatinib) package insert. Princeton, NJ: Bristol-Myers Squibb Co; September 2016.
35. Gleevec (imatinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; September 2016.
36. Tasigna (nilotinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; September 2016.
37. Votrient (pazopanib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; August 2016.
38. Stivarga (regorafenib) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; June 2016.
39. Nexavar (sorafenib) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals; June 2015.
40. Sutent (sunitinib maleate) package insert. New York, NY: Pfizer Inc; May 2015.
41. Bramwell V, Rouesse J, Steward W, et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma—reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1994;12:1137-1149.
42. Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537-1545.
43. Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2676-2684.
44. Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144-3150.
45. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of Sarcoma Alliance for Research through Collaboration study 002. J Clin Oncol. 2007;25:2755-2763.
46. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
47. Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23:1680-1687.
48. Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045-1054.
49. Blay JY, Leahy MG, Nguyen BB, et al. Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur J Cancer. 2014;50:1137-1147.
50. Blay JY, Shen L, Kang YK, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol. 2015;16:550-560.
To comment on this article, contact rdavidson@uspharmacist.com.






