US Pharm. 2018;43(7):43-44.
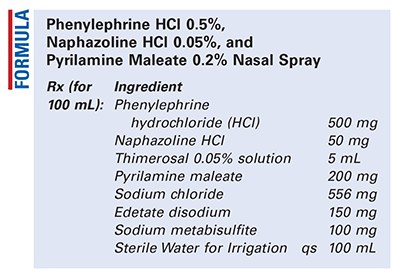
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh and/or measure each ingredient. In a 250-mL beaker, dissolve the phenylephrine hydrochloride (HCl), naphazoline HCl, and pyrilamine maleate in 40 mL of Sterile Water for Irrigation. In a separate beaker, dissolve the edetate disodium in warm Sterile Water for Irrigation (48 mL). Allow to cool and then dissolve the sodium metabisulfite and sodium chloride in the solution. Mix together the two solutions. Add the thimerosal solution and bring to volume with Sterile Water for Irrigation. Package in nasal-spray bottles and label.
Packaging: Package in tight, light-resistant nasal-spray containers.
Labeling: Keep out of reach of children. Use only as directed. For the nose.
Use: This preparation is used as a nasal decongestant with antihistaminic properties.
Stability: A beyond-use date of up 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, clarity, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, mold growth).2
Discussion: Phenylephrine HCl (Neo-Synephrine, C9H13NO2.HCl, MW 203.67) is a sympathomimetic amine that acts predominantly by a direct effect on alpha-adrenergic receptors. It occurs as white or practically white, odorless crystals with a bitter taste and is freely soluble in water and alcohol. Commercially, phenylephrine HCl injections may contain an antioxidant (sodium metabisulfite) and the air has been replaced with nitrogen to prevent oxidation. Phenylephrine HCl is incompatible with ferric salts, oxidizing agents, or metals.1
Naphazoline HCl (Naphcon, Vasocon, C14H14N2.HCl, MW 246.74) occurs as a white, crystalline powder that is freely soluble in water and soluble in alcohol. It is an adrenergic, vasoconstrictor, and nasal decongestant.1
Thimerosal is an antibacterial agent with weak bacteriostatic and mild fungistatic properties. It is relatively nontoxic and is available as a powder and a solution. The powder is light cream–colored and crystalline, with a slight characteristic odor. Thimerosal is soluble 1 g in about 1 mL of water and approximately 12 mL of alcohol. The pH of a 1% solution is about 6.7. Thimerosal is affected by light.3
Pyrilamine maleate (C17H23N3O.C4H4O4, MW 401.46) occurs as a white, crystalline powder, usually with a faint odor. It is highly soluble in water and freely soluble in alcohol. Its aqueous solutions are acid to litmus. Pyrilamine maleate is used for its antihistaminic properties.1
Edetate disodium (edetate disodium, edetic acid disodium, disodium ethylenediaminetetraacetate, C10H14N2Na2O8, MW 336.21) occurs as an odorless, white, crystalline powder with a slightly acid taste. It is soluble 1 g in 11 mL of water, slightly soluble in 95% ethanol, and practically insoluble in chloroform and ether. The pH of a 1% w/v solution in carbon dioxide–free water is in the range of 4.3 to 4.7. Edetate disodium also occurs as a dihydrate (C10H14N2Na2O8.2H2O, MW 372.2). The pH of the USP injection ranges from 6.5 to 7.5. Edetate disodium melts at 252°C, with some decomposition. It forms a stable soluble complex with calcium and has been used in the treatment of deposits from calcium oxide or calcium hydroxide burns of the eye; it also has been used to treat corneal opacities, either by topical application or by iontophoresis.4
Sodium metabisulfite (disodium disulfite, Na2S2O5, MW 190.1) is an antioxidant occurring as colorless, prismatic crystals or as a white to creamy-white, crystalline powder that has the odor of sulfur dioxide and an acidic, saline taste. It is soluble 1 g in 1.9 mL of water, slightly soluble in 95% ethanol, and freely soluble in glycerin. The pH of a 5% w/v aqueous solution is in the range of 3.5 to 5.0. Sodium metabisulfite is used as an antioxidant in oral, parenteral, and topical pharmaceutical formulations. Generally, sodium metabisulfite is used at low pH, sodium bisulfite at intermediate pH, and sodium sulfite at higher pH values. In water, sodium metabisulfite is immediately converted to sodium (Na+) and bisulfite (HSO3–) ions.5
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; June 2018.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Kadri BV. Thimerosal. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:974-977.
4. Cantor SL, Shah S. Disodium edetate. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:347-349.
5. Cook W, Foan ES. Sodium metabisulfite. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:873-875.
To comment on this article, contact rdavidson@uspharmacist.com.






