US Pharm. 2017;42(7):27-37.
ABSTRACT: Tobacco use among adults and adolescents remains prevalent in the United States. Many smokers wish to quit, but a number of cessation attempts are usually necessary to overcome tobacco use. Tobacco use should be treated as a chronic condition, and the community pharmacist must assess the patient’s readiness to quit prior to initiating pharmacotherapy. Behavioral interventions should be utilized in the patient who is not ready to quit, and these should be coupled with an appropriate pharmacotherapy selection once the patient is ready to quit. Community pharmacists are well positioned to perform interventions with patients who use tobacco.
Tobacco use is the leading preventable cause of death in the United States.1 The CDC reports that the rate of smoking in adults, which has steadily declined from 43% in 1964—when the Surgeon General first warned of the health risks associated with smoking—is 16.8%.2 Tobacco use is decreasing in adolescents after a brief spike in the early 2000s; the rate is currently 16.7%.2 There is room for improvement in reaching Healthy People 2020 smoking-reduction objectives of 12% and 16% in adults and adolescents, respectively.2,3 It is well established that tobacco use—including both combustible and smokeless tobacco—can increase a person’s risk of developing several conditions, including lung cancer, coronary artery disease, and stroke, as well as increase overall mortality.2 It is also important to address tobacco use during pregnancy because of complications including preterm delivery, low birthweight, cleft lip, and stillbirth.4
Despite the awareness of associated harm, current smokers face a significant challenge in quitting smoking that is attributed to nicotine’s potent euphoriant effects.5 Nicotine exerts its effects via binding of nicotinic receptors in the central nervous system, causing a release of dopamine. Dopamine triggers areas in the brain that control pleasure, reward, and compulsive behaviors, leading to the smoker’s dependence on nicotine-triggered dopamine release.6 Continual stimulation of the pleasure center of the brain is what makes smoking cessation so challenging.
Identifying Level of Dependence and Attitudes Toward Cessation
Pharmacists should ask about tobacco use at every encounter.7,8 It is important to phrase the question so that both smokeless tobacco use and occasional use can be identified. Although this is not commonly practiced in the community-pharmacy setting, tobacco use should be documented in the patient’s profile. When verifying prescriptions, community pharmacists can use this information to identify intervention opportunities. Pharmacists should also be aware that not all patients who are inquiring about smoking cessation or who have a prescription for a smoking-cessation product are ready to quit.9 Behavioral interventions should be performed to identify the patient’s stage of quitting in order to determine the next step.9
The first phase of intervention is to employ the 5A’s (steps) of smoking cessation (TABLE 1).7 The pharmacist should be mindful of how questions are phrased. In the Ask step, simply querying, “Are you a smoker?” may miss light or occasional smokers. Patients should always be approached with empathy and support and in a nonjudgmental manner.7 In the Assess step, if the patient is not ready to quit, the pharmacist should move to the transtheoretical model of change (TABLE 2).7-10 The patient must progress through a series of behaviors before he or she is deemed ready to quit.9 The 5R’s of smoking cessation (relevance, risks, rewards, roadblocks, repetition) should be incorporated in the precontemplative or contemplative stage.7 For long-term success, the decision to quit must be the patient’s.11 The pharmacist should not initiate smoking-cessation therapy if the patient is not ready.

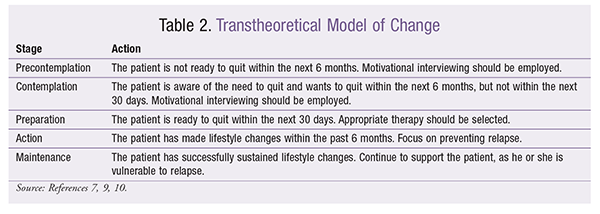
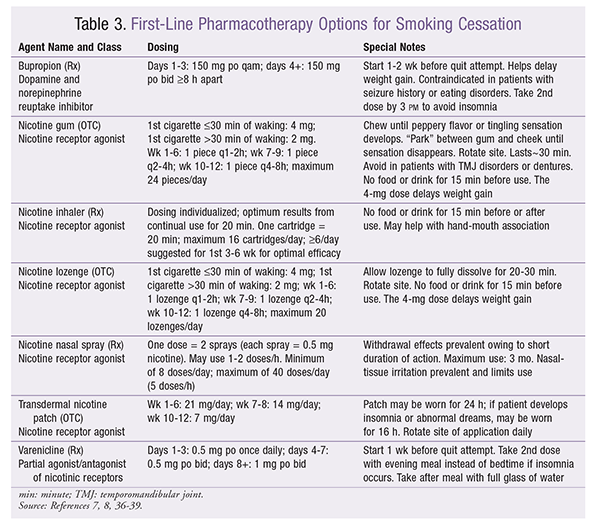
TABLE 3 summarizes first-line therapies for smoking cessation.7,8 Pharmacologic interventions are recommended for patients who smoke 10 or more cigarettes per day.7,8 It is important to note that cessation rates are higher with a combination of pharmacotherapy and nonpharmacologic treatment. Nonpharmacologic treatments include behavioral counseling, massage, acupuncture, hypnosis, and other methods.9 Behavioral interventions alone should be used in patients smoking less than 10 cigarettes per day.7,8 In December 2016, the FDA removed the Black Box Warning about neuropsychiatric events for both bupropion and varenicline.12 This was prompted by the high comorbidity of mental-health conditions in smokers, as well as by the EAGLES trial, which showed no significant increase in neuropsychiatric adverse events (AEs) attributable to varenicline or bupropion.13 History of mental-health conditions has a stronger association than the medications themselves.13 Pharmacists should consider these medications in patients with mental-health conditions; however, close monitoring for neuropsychiatric events is still warranted.
Clonidine and nortriptyline are second-line agents because they are not FDA-approved for smoking cessation, they have the potential for undesirable AEs, and they have less evidence as smoking-cessation agents.7 They should be considered when a patient cannot use, or fails, first-line treatments.7
Among first-line agents, guidelines do not preferentially recommend a therapy. Pharmacists should consider a therapy with long-acting minimization of withdrawal symptoms (bupropion, transdermal nicotine patches, varenicline), along with a short-acting therapy (nicotine gum, nicotine lozenge) to help with breakthrough cravings. Generally, the nicotine inhaler and nicotine nasal inhaler are poorly tolerated, and there is less evidence of their efficacy and role. Although combination therapy has been found to provide initial improvement in cessation rates, long-term rates at 6, 12, and 24 months are similar to rates with monotherapy.14 However, incidences of AEs are similar, so these agents may be used if the patient feels more prepared to quit with combination therapy.14
Varenicline produces the highest cessation rates; bupropion and combination nicotine replacement therapy (NRT) are the next most effective agents.7,8,15 No difference in effectiveness has been found among NRTs; however, nicotine lozenges deliver 25% more nicotine than nicotine gum, owing to quicker medication release despite similar pharmacokinetics.7-9,15 Combination NRT is more effective than single NRT.15 Duration of use has been associated with prolonged cessation success.16 For both NRT and prescription medications, use for 5 weeks or more leads to greater success.16 Unique dosing strategies, primarily related to varenicline, are emerging. Studies of preloading varenicline 4 weeks prior to the quit date to induce a greater reduction in smoking enjoyment have shown promise.17 This strategy reduced smoking enjoyment and the number of cigarettes smoked prior to the quit date and resulted in improved cessation rates at 12 weeks.17 The use of varenicline to reduce the number of cigarettes smoked in patients unwilling or unable to quit in the next month also achieved better cessation rates at 24 weeks and 1 year without any significant difference in serious AEs.18 Pharmacists may consider these dosing strategies in patients with significant quitting fears within 1 week or in those who have struggled with previous quit attempts.
Electronic Cigarettes: a Role in Smoking Cessation?
Electronic cigarettes (e-cigarettes) are battery-operated devices containing nicotine and other substances (e.g., propylene glycol). E-cigarettes are growing in popularity: There were 2.4 million users in middle school and high school in 2014, and 12.6% of adult smokers have tried them.19 Those most likely to use e-cigarettes are young adults (age 18-24 years) and current smokers who attempted to quit in the past year.19 These devices have provoked safety concerns, and their role in smoking cessation is heavily debated. E-cigarettes have been found to emit toxic compounds, such as formaldehyde, via breakdown of cartridge substances (e.g., propylene glycol, glycerin).20 Although they are thought to emit significantly lower levels of carcinogens than tobacco cigarettes, they have been shown to emit toxic chemicals (e.g., diethylene glycol) and carcinogenic impurities (e.g., N-nitrosonornicotine).21 Diethylene glycol, a flavoring agent previously used to manufacture popcorn, is associated with the development of bronchiolitis obliterans (“popcorn lung”), a severe condition involving permanent loss of lung function.22 A study of 51 e-cigarette flavoring juices (e-juices) found diethylene glycol in 76% of e-juices, raising concerns over health risks different from those of combustible cigarettes.22 E-cigarette flavorings have been identified as a potential harm by the American Lung Association.23
Recent evidence regarding the efficacy of e-cigarettes for smoking cessation is conflicting.24-27 A systematic review and meta-analysis found that smokers using e-cigarettes as a cessation aid were 28% less likely to quit, whereas other studies have shown short-term, but not long-term, efficacy of e-cigarettes as a cessation aid.25,26 In a comparison of e-cigarettes and tobacco cigarettes, there was no significant difference in abstinence from tobacco after 24 months.27 Patients using e-cigarettes may reduce the number of cigarettes smoked per day; however, they rarely abstain from the use of tobacco products, instead becoming dual users.27
Controversy has followed e-cigarettes ever since they entered the market. Since August 8, 2016, the FDA has had the authority to regulate all tobacco products, including e-cigarettes.28 The FDA’s tobacco rule creates significant access restrictions and requires regulations and testing to verify the contents of e-juice.28 The pharmacist should assess the patient’s use of e-cigarettes in place of combustible cigarettes or as a cessation aid. Despite the lack of consistent evidence for e-cigarettes as a cessation aid, physicians support their use 61% of the time when asked by patients who are interested in quitting.29 Based on inconsistent evidence and the potential for emerging health risks, e-cigarettes are not an appropriate cessation tool for most patients. Their use should be restricted to patients who will not attempt any other cessation method, and patients should be apprised of the potential harms.
Community Pharmacists as Smoking-Cessation Treatment Specialists
As the most accessible healthcare providers, community pharmacists can support patients’ attempts to quit smoking. A systematic review of tobacco interventions by pharmacists demonstrated that pharmacists can deliver smoking-cessation services and suggested they are effective in helping patients successfully quit.30 Community pharmacists who provide smoking-cessation services have cessation rates similar to those of other healthcare professionals.31 The pharmacist should select the most appropriate therapy for the patient and counsel the patient on how to use it correctly.11 It is estimated that a smoker requires eight to 11 cessation attempts, and a Gallup poll reported that smokers average 6.1 quit attempts before achieving success.32,33 However, recent literature indicates that the average may be as high as 30 quit attempts, noting that previous figures may be underestimated because of the exclusion of smokers facing significant challenges in quitting and limitations in smokers’ ability to recall lifetime quit attempts.34
Pharmacists should be encouraged to perform interventions when counseling patients who are picking up medications; even a brief intervention (3 minutes) increases patient interest and success in quitting.9 Counseling interventions have been associated with greater cessation success as the number of interventions increases.9 Community pharmacists should be supportive of patients who are unsuccessful, identifying barriers and adjusting pharmacotherapy. It should be expected that smokers will require multiple attempts, and community pharmacists should assist in both behavioral counseling and pharmacotherapy management. All patients should be referred to 1-800-QUIT-NOW for additional support. Pharmacists can also use the quizzes at Smokefree.gov (e.g., a quiz on identifying stressors and how to manage them; a withdrawal quiz to determine the degree of symptoms; and a nicotine-addiction quiz that assesses how much the patient relies on nicotine and ways to curb cravings).35
The community pharmacist’s ability to intervene varies by state of practice and by practice workflow and volume. Many states allow pharmacists to enter collaborative-practice agreements wherein a pharmacist can initiate therapy or assess cessation success and monitor AEs, allowing continued support and collaboration with the prescriber for plan modification. Even in more restrictive states or at high-volume sites, when counseling patients on chronic medications and disease states, the pharmacist may use brief interventions to encourage them to consider quitting smoking, in order to support their progress toward cessation.
Conclusion
Tobacco use is a chronic condition that requires patient persistence and motivation to succeed, along with behavioral interventions (e.g., motivational intervention) by pharmacists. Community pharmacists are well positioned to offer smoking-cessation services, given their frequent contact with patients. To optimize cessation attempts and increase the number of patients interested in cessation, community pharmacists must integrate appropriate pharmacotherapy and behavioral interventions. The use of e-cigarettes for smoking cessation is growing among patients; however, data are mixed regarding the success of this device as a cessation tool, and its status as a risk-reduction strategy is controversial.
REFERENCES
1. CDC. Health effects of cigarette smoking. www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/. Accessed December 26, 2016.
2. CDC. Trends in current cigarette smoking among high school students and adults, United States, 1965-2014. www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/. Accessed March 1, 2017.
3. HealthyPeople.gov. Tobacco use. www.healthypeople.gov/2020/topics-objectives/topic/tobacco-use/objectives. Accessed March 10, 2017.
4. WHO. Fact sheet about health benefits of smoking cessation. www.who.int/tobacco/quitting/benefits/en/. Accessed December 28, 2016.
5. Rennard SI, Daughton DM. Cigarette smoking and smoking cessation. In: Grippi MA, Elias JA, Fishman JA, et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill Education; 2015.
6. Vijayaraghavan M, Schroeder SA. Tobacco use. In: King TE, Wheeler MB, eds. Medical Management of Vulnerable and Underserved Patients: Principles, Practice, and Populations. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
7. Agency for Healthcare Research and Quality. Clinical guidelines for prescribing pharmacotherapy for smoking cessation. www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/prescrib.html. Accessed June 6, 2017.
8. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
9. Hudmon KS, Corelli RL, Kroon LA, et al. Reducing pulmonary disease: the pharmacist’s role in smoking cessation. J Pharm Pract. 2001;24:143-159.
10. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48.
11. Kim C, Shim J, Avena-Woods C. Smoking cessation and the pharmacist-patient relationship. Drug Topics. http://drugtopics.modernmedicine.com/drug-topics/content/tags/cigarettes/smoking-cessation-and-pharmacist-patient-relationship?page=full. Accessed March 1, 2017.
12. FDA. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. www.fda.gov/Drugs/DrugSafety/ucm532221.htm. Accessed March 1, 2017.
13. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507-2520.
14. Ternullo SR, Abdolahi A, Williams GC. Study of monotherapy versus combination therapy for tobacco dependence among heavily addicted smokers. J Am Pharm Assoc (2003). 2017;57:77-81.e1.
15. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis (review). Cochrane Database Syst Rev. 2013;(5):CD009329.
16. Siahpush M, Shaikh RA, McCarthy M, et al. Association between duration of use of pharmacotherapy and smoking cessation: findings from a national survey. BMJ Open. 2015;5:e006229.
17. Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171:770-777.
18. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
19. CDC. No decline in overall youth tobacco use since 2011. www.cdc.gov/media/releases/2016/p0414-youth-tobacco.html. Accessed March 1, 2017.
20. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1390-1326.
21. Wynn WP III, Stroman RT, Almgren MM, Clark KJ. The pharmacist “toolbox” for smoking cessation: a review of methods, medicines, and novel means to help patients along the path of smoking reduction to smoking cessation. J Pharm Pract. 2012;25:591-599.
22. Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733-739.
23. American Lung Association. E-cigarettes and lung health. www.lung.org/stop-smoking/smoking-facts/e-cigarettes-and-lung-health.html. Accessed December 28, 2016.
24. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:116-128.
25. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
26. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
27. Manzoli L, Flacco ME, Ferrante M, et al. Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tob Control. 2016;1-9.
28. FDA. FDA’s new regulations for e-cigarettes, cigars, and all other tobacco products. www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm394909.htm. Accessed June 6, 2017.
29. Kollath-Cattano C, Thrasher JF, Osman A, et al. Physician advice for e-cigarette use. J Am Board Fam Med. 2016;29:741-747.
30. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27:1040-1051.
31. Shen X, Bachyrycz A, Anderson JR, et al. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20:579-587.
32. American Cancer Society. How to quit smoking or smokeless tobacco. www.cancer.org/healthy/stay-away-from-tobacco/guide-quitting-smoking.html. Accessed June 6, 2017.
33. Jones JM. Smoking habits stable; most would like to quit. www.gallup.com/poll/23791/smokinghabits-stable-most-would-like-quit.aspx. Accessed March 1, 2017.
34. Chaiton M, Diemert L, Cohen JE, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6:e011045.
35. Smokefree.gov. Quizzes. www.smokefree.gov/quizzes. Accessed March 1, 2017.
36. Mospan CM. Updates in pharmacotherapy for smoking cessation. J Nurse Pract. 2016;12:421-422.
37. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. http://online.lexi.com. Accessed March 1, 2017.
38. FDA. Nicorette gum labeling. www.accessdata.fda.gov/drugsatfda_docs/label/2012/018612s061_020066s042lbl.pdf. Accessed March 1, 2017.
39. FDA. Nicorette mini lozenge labeling. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022360Orig1s007lbl.pdf. Accessed March 1, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.






