US Pharm. 2019;44(2):16-24.
ABSTRACT: Elevated cholesterol, along with other risk factors, predisposes patients to increased cardiovascular disease and events. In November 2018, the American College of Cardiology and the American Heart Association published updated cholesterol guidelines that stratify pharmacotherapy for patients considered to be at very high risk for cardiovascular disease. Zetia (ezetimibe) and the PCKS9 inhibitors Praluent (alirocumab) and Repatha (evolocumab) may be used after maximally tolerated statin therapy. The use of these medications has been shown to reduce cholesterol and decrease mortality, and community pharmacists are well positioned to help maximize pharmacotherapy in patients with elevated cholesterol.
Elevated total and LDL cholesterol predispose patients to an increased risk of cardiovascular disease and mortality. An estimated 42.8% of Americans aged older than 20 years have a total cholesterol level exceeding 200 mg/dL. Unless patients have experienced a cardiovascular event, they remain asymptomatic. Notably, only about one-third of treated patients achieve their LDL goal, and even fewer patients with coronary heart disease reach their target LDL level.1
Updated Cholesterol Guidelines
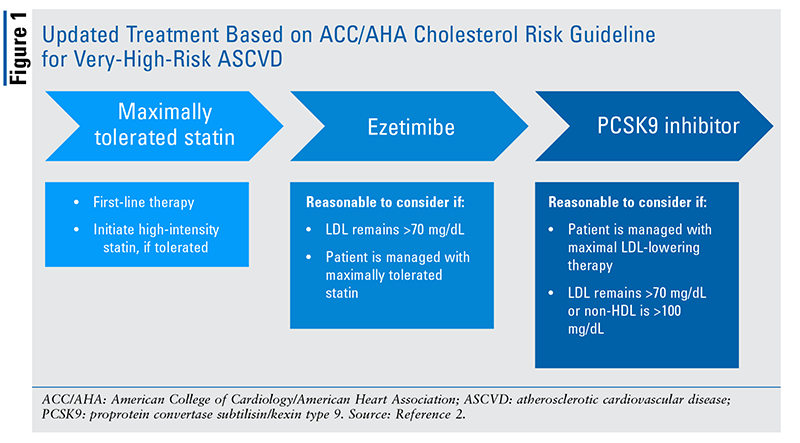
In November 2018, the American College of Cardiology and the American Heart Association (ACC/AHA) published updated guidelines focusing on new pharmacologic therapy for hyperlipidemia.2 The update recommends earlier screening in patients with a family history of heart disease or high cholesterol. Additionally, although 50% LDL cholesterol lowering is still the goal for patients treated with a high-intensity statin, an LDL goal of less than 70 mg/dL warrants consideration of ezetimibe followed by proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in patients who have not sufficiently lowered their LDL. Populations benefited by statins remain relatively unchanged, except that PCSK9 inhibitors are recommended for secondary prevention with a maximally tolerated statin in patients at very high risk for cardiovascular disease (defined as a history of multiple cardiovascular events or one major event and multiple high-risk conditions). FIGURE 1, a treatment algorithm based on the 2018 ACC/AHA guideline for the management of cholesterol, details when PCSK9 inhibitors should be considered in patients at very high risk.2
Statins
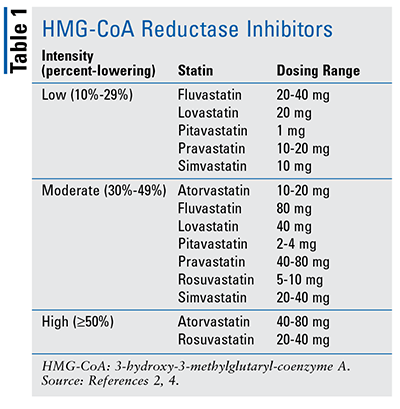
Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, an enzyme responsible for converting HMG-CoA into mevalonic acid, a cholesterol precursor. Additionally, statins alter the conformational shape of the enzyme at its active site, leading to a reduction of intracellular cholesterol and, ultimately, an increase in hepatic LDL receptors.3 Statins range in intensity from low to moderate to high, depending on the amount of LDL lowering warranted and the patient’s cardiovascular history (TABLE 1).2,4
An extensive amount of research supports the use of statins for secondary prevention.5-7 In the PROVE IT-TIMI 22 trial, 4,162 patients received pravastatin 40 mg or atorvastatin 80 mg within 10 days of hospitalization for acute coronary syndrome. The primary outcome was time to the first component of the composite endpoint, including death from any cause, myocardial infarction, documented unstable angina requiring either percutaneous coronary intervention or coronary-artery bypass grafting, and stroke. Over 2 years, 26.3% of patients receiving pravastatin and 22.4% of those receiving atorvastatin met the primary outcome (hazard ratio [HR] 0.84; 95% CI, 0.05-0.26; P = .005).7
In an examination of primary prevention, the MEGA study assessed treatment with pravastatin 10 mg initially plus diet versus diet alone in 7,832 patients followed for an average of 5.3 years.8 The primary outcome was the first occurrence of coronary heart disease. Patients included men and postmenopausal women aged 40 to 70 years with hypercholesterolemia and a total body weight of 40 kg or greater. Patients at increased risk for cardiovascular events had a history of hypertension (42%) or diabetes (21%) or were current or previous smokers (20.5%). Patients who took pravastatin in combination with a diet were 33% less likely than the diet-alone group to experience coronary heart disease over a 4-year period (HR 0.67; 95% CI, 0.49-0.91; P = .01). This benefit was seen despite only an 18% reduction in LDL in the pravastatin-plus-diet group versus a 3% reduction in LDL in the diet-alone group over 5.3 years (P <.0001).8
Recommendations for statin use remain relatively unchanged since the 2013 and 2016 ACC/AHA cholesterol guidelines.9,10 Statin therapy continues to be the treatment of choice for both primary and secondary prevention. Myalgia remains the most commonly reported adverse effect with statins. New-onset diabetes has also been observed in clinical trials, but more commonly in patients with increased diabetes risk. The negative effects of statins on memory and cognition have been noted anecdotally in many case reports but have not been substantiated in larger trials.2
Ezetimibe
Zetia (ezetimibe) impedes intestinal absorption of cholesterol through inhibition of the sterol transporter Niemann-Pick C1 Like 1. The reduced absorption of cholesterol leads to a decrease in the delivery of intestinal cholesterol to the liver, thereby reducing overall blood cholesterol levels.11 Ezetimibe is indicated for use as monotherapy in patients with primary hyperlipidemia. Ezetimibe is also indicated for use as combination therapy with statins in patients with primary hyperlipidemia and those with homozygous familial hypercholesterolemia.
The SHARP trial assessed 9,270 patients with chronic kidney disease (3,023 on dialysis) given either simvastatin plus ezetimibe or simvastatin alone who were followed for an average of 4.9 years.12 The primary outcome was the first major atherosclerotic event (defined as nonfatal myocardial infarction or coronary death, nonhemorrhagic stroke, or any arterial revascularization procedure). The simvastatin-ezetimibe group was less likely than the simvastatin-alone group to experience a major atherosclerotic event (rate ratio [RR] 0.83; 95% CI, 0.74-0.94; P = .0021). In addition, simvastatin-ezetimibe patients were less likely to experience major vascular events (i.e., major atherosclerotic events plus noncoronary cardiac deaths and hemorrhagic stroke; 15.1% vs. 17.6%; RR 0.85; 95% CI, 0.77-0.94; P = .0012). Although investigators found a statistically significant reduction in these endpoints, there was no statistical difference between groups in nonfatal myocardial infarction or coronary heart disease mortality (4.6% vs. 5%; RR 0.92; 95% CI, 0.77-1.11; P = .37).12
IMPROVE-IT randomized 18,144 patients older than age 50 years to receive either simvastatin 40 mg plus ezetimibe 10 mg or monotherapy with simvastatin 40 mg.13 Patients had been hospitalized within the past 10 days for acute coronary syndrome. The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization, and nonfatal stroke. Patients were followed for a median of 6 years. The primary-outcome event rate at 7 years in the simvastatin-ezetimibe group was 32.7%, versus 34.7% in the simvastatin-monotherapy group (HR 0.936; 95% CI, 0.89-0.99; P = .016).13
SHARP and IMPROVE-IT data showing decreased adverse cardiac events prompted the newest update of the ACC/AHA lipid guidelines to include recommendations for the use of nonstatin therapy, including ezetimibe.12,13 Ezetimibe is now recommended to be added to a patient’s statin therapy if the patient is older than age 75 years, does not have a very high risk of atherosclerotic cardiovascular disease (ASCVD), and does not achieve LDL lowering of 50% or more on a maximally tolerated statin. Additionally, it is recommended to add ezetimibe to high-intensity statin therapy in patients with very-high-risk ASCVD whose LDL is greater than 70 on statin therapy. Interestingly, ezetimibe is recommended over PCSK9 inhibitors to further reduce LDL, but this may be due primarily to the high cost of PCSK9 inhibitors.2
PCSK9 Inhibitors
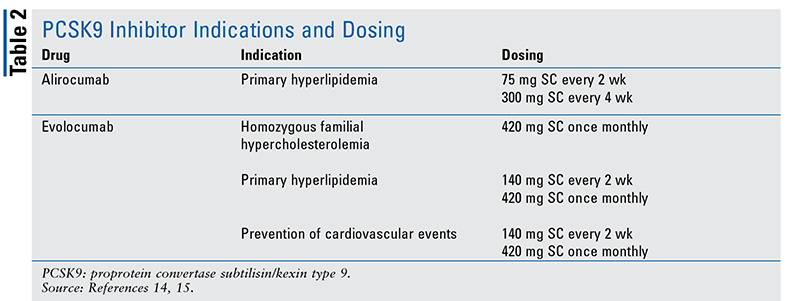
Approved by the FDA in July 2015 and August 2015, respectively, Praluent (alirocumab) and Repatha (evolocumab) are monoclonal antibodies that inhibit PCSK9.14,15 PCSK9 binds to LDL receptors on the surface of hepatocytes, thereby degrading the LDL receptor. When PCSK9 is inhibited, LDL receptors remain active and available on hepatocytes’ surfaces to clear LDL from the blood. Both alirocumab and evolocumab are indicated for primary hyperlipidemia. Additionally, evolocumab is indicated for prevention of cardiovascular events and homozygous familial hypercholesterolemia (TABLE 2).14,15
The ODYSSEY LONG TERM trial compared alirocumab with use of a high-intensity or maximally tolerated statin to evaluate the percentage change in LDL cholesterol over a 24-week period.16 Included were 2,341 patients aged 18 years and older with heterozygous familial hypercholesterolemia or established coronary artery disease. Patients were already receiving a high-intensity statin or were taking a statin at the maximally tolerated dose for at least 4 weeks prior to screening. Maximally tolerated was defined as the highest possible dose with an acceptable side-effect profile. Notably, only 46.8% of patients were receiving a high-intensity statin at baseline. The average decrease in LDL cholesterol was 62% in patients receiving alirocumab (P <.001).16
The recent ODYSSEY OUTCOMES trial randomized 18,924 patients aged 40 years and older to receive alirocumab 75 mg or placebo every 2 weeks.17 Patients had experienced an acute coronary syndrome within 1 to 2 months prior to randomization and were managed on either a high-intensity statin or a maximally tolerated statin. The primary endpoint was the composite of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. Patients were followed for a median of 2.8 years. The primary composite endpoint occurred in 9.5% of patients receiving alirocumab compared with 11.1% in patients receiving placebo (HR 0.85; 95% CI, 0.78-0.93; P <.001). The secondary endpoint of all-cause mortality was also significantly different in patients who received alirocumab than in those who received placebo (P = .026).17
The OSLER trials compared evolocumab plus standard therapy with standard therapy alone to evaluate the percent change from baseline in LDL over a 1-year period.18 Similar to what was seen in ODYSSEY LONG TERM, LDL cholesterol decreased 61% in patients with familial heterozygous hypercholesterolemia who received evolocumab plus standard therapy (P <.001).18 More recently, however, the FOURIER trial compared evolocumab with high-intensity statin therapy to investigate cardiovascular benefit.19 Patients were aged 40 to 85 years, had ASCVD, and preferably were taking a high-intensity statin. In this trial, similar LDL reduction was seen with evolocumab as in the OSLER trials (P <.0001); however, there was also a significant reduction in the composite endpoint of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization (HR 0.85; 95% CI, 0.79-0.92; P <.001). Because this study was conducted over 1 year, long-term mortality data were not obtained.19
Although it caused impressive LDL lowering, evolocumab failed to show a benefit for mortality outcomes given the brief length of the most recent FOURIER trial and its follow-up period.19 Based on the results of the ODYSSEY OUTCOMES trial, alirocumab has shown a significant benefit for all-cause mortality versus placebo.18 Both alirocumab and evolocumab resulted in reductions in cardiovascular outcomes with significant LDL lowering. As previously mentioned, the latest cholesterol guidelines recommend the addition of PCSK9 inhibitors after sufficient LDL lowering has not been achieved with a high-intensity statin and ezetimibe. Specifically, the guidelines recommend initiation of a PCSK9 inhibitor if LDL remains greater than 70 mg/dL.2
The benefit of PCSK9 inhibitors must be weighed against these drugs’ substantial financial burden. Although the cost has decreased since the drug gained FDA approval in 2015, the annual cost of either alirocumab or evolocumab ranges from $5,900 to $14,500.20 Generally well tolerated with the exception of injection-site reactions, both alirocumab and evolocumab have shown remarkable promise as an option for cholesterol lowering in high-risk or very-high-risk patients who can afford this therapy.
Implications for Community Pharmacy
Pharmacists are positioned to recommend additional cholesterol-lowering therapies to further reduce cardiovascular outcomes and mortality in high-risk patients. The latest cholesterol guidelines reinforce statin therapy as the cornerstone of pharmacotherapy. Community pharmacists can assist both patients and providers in selecting and obtaining additional agents—ezetimibe or PCSK9 inhibitors—while considering the financial cost to the patient.
REFERENCES
1. Talbert RL. Dyslipidemia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill; 2017.
2. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. S0735-1097(18)39034-x [Epub ahead of print].
3. Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378-387.
4. Chou R, Dana T, Blazina I, et al. Table 1: statin dosing and ACC/AHA classification of intensity. In: Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016. www.ncbi.nlm.nih.gov/books/NBK396417/table/ch1.t1/. Accessed December 13, 2018.
5. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335(14):1001-1009.
6. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357.
7. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504.
8. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163.
9. Robinson JG. Overview of the 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Future Cardiol. 2014;10(2):149-152.
10. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725.
11. Zetia (ezetimibe) package insert. Whitehouse Station, NJ: Merck & Co, Inc; August 2013.
12. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192.
13. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397.
14. Praluent (alirocumab) package insert. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; November 2018.
15. Repatha (evolocumab) package insert. Thousand Oaks, CA: Amgen, Inc; October 2018.
16. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-1499.
17. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107.
18. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509.
19. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722.
20. Turgeon RD, Pearson GJ. Proprotein convertase subtilisin/kexin type 9 inhibitors for reduction of cardiovascular events. Am J Health Syst Pharm. 2018;75(11):747-754.
To comment on this article, contact rdavidson@uspharmacist.com.





