US Pharm. 2017;42(2):HS15-HS18.
ABSTRACT: Hyperkalemia (elevated serum potassium) can become a life-threatening electrolyte abnormality due to medication use, kidney dysfunction, or alternative sources of electrolyte imbalance. Up until recently, FDA-approved therapies for the management of hyperkalemia (i.e., sodium polystyrene sulfonate) had remained unchanged for over 50 years. Other treatment options for hyperkalemia include IV calcium, insulin, sodium bicarbonate, albuterol, and diuretics. A new drug (patiromer) was recently approved for the treatment of hyperkalemia, and additional agents are also in development.
Hyperkalemia is defined as a serum potassium concentration of >5.5 mEq/L in adults.1 It is a common metabolic disorder that can lead to clinical manifestations such as hemodynamic instability, neurologic sequelae, and fatal arrhythmias. Most individuals with hyperkalemia are usually asymptomatic or present with nonspecific signs and symptoms (e.g., weakness, fatigue, or gastrointestinal [GI] hypermotility). The incidence of hyperkalemia has been reported anywhere from 2.6% to 3.2% in the United States.2,3 A study in Canada showed the incidence to occur in 2.6% of emergency department visits and 3.5% of hospital admissions.4
Hyperkalemia is commonly a result of impaired urinary potassium excretion due to acute or chronic kidney disease (CKD), reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery, effective arterial blood volume depletion, or selective impairment in potassium secretion. Hyperkalemia can also occur secondarily to metabolic acidosis, insulin deficiency, hyperglycemia, and hyperosmolar states. Medication can also lead to hyperkalemia, most notably those agents that inhibit the renin-angiotensin-aldosterone system (RAAS). Other drugs with the potential to cause hyperkalemia include beta-blockers, succinylcholine, trimethoprim-sulfamethoxazole, non-steroidal anti-inflammatory drugs (NSAIDs), cyclosporine, heparins, tacrolimus, and excessive dosing of potassium supplements. Overdoses of digitalis or related digitalis glycosides, such as digoxin, can also lead to hyperkalemia. Salt substitutes (e.g., Mrs. Dash) are often overlooked as a cause of hyperkalemia.5
Therapy for hyperkalemia due to potassium retention includes avoiding drugs that potentially induce hyperkalemia, discontinuing offending agents such as potassium supplements, and ultimately inducing potassium loss.5
Treatment Options
Acute hyperkalemia is a clinical emergency that requires immediate treatment with the agents discussed below (TABLE 1).
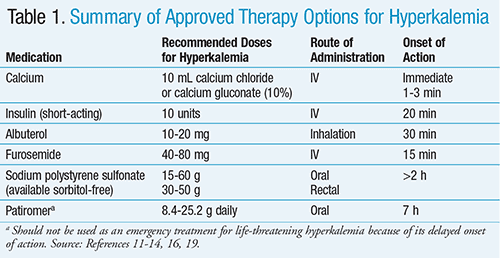
IV Calcium: IV calcium is indicated when the serum potassium is >6.5 mEq/L regardless of whether ECG changes are present.6 Given their poor sensitivity and specificity, ECG changes should not be used as diagnostic criteria for treatment of hyperkalemia.7 The immediate goal of acute management in hyperkalemia is the stabilization of the membrane potential, which is often done with IV calcium. Calcium antagonizes the effects of hyperkalemia through effects on the threshold potential and the speed of impulse propagation at the cellular level. IV calcium can be given as 10 mL of a 10% calcium gluconate solution over 2 to 3 minutes or as calcium chloride, which contains three times the amount of calcium per 10 mL dose. Calcium chloride is also irritating to the veins and must be given via central catheters. It is important to note that IV calcium is administered for myocardium membrane stabilization and does not promote the intracellular shift or elimination of potassium.6
Insulin: Insulin accelerates the intracellular movement of potassium into muscle cells by binding to its receptor on skeletal muscle. Once this occurs, the abundance and activity of sodium-potassium adenosine triphosphatase (Na+/K+-ATPase) and glucose transporter on the cell membrane increase through independent signaling pathways. The most commonly recommended regimen is a bolus injection of short-acting insulin. If the blood glucose is <250 mg/dL, 25 g of glucose should also be given (50 mL of a 50% solution) to offset hypoglycemia due to insulin administration.8-10
Sodium Bicarbonate: Sodium bicarbonate works to shift potassium intracellularly but is not considered first-line management of hyperkalemia due to controversial data regarding efficacy and safety concerns. Boluses of 1 mL/kg of sodium bicarbonate 8.4% solution have been suggested.10 Studies have shown that sodium bicarbonate was not able to decrease serum potassium significantly or rapidly, with onset of action potentially taking hours.11,12 Its use in patients with metabolic acidosis and hyperkalemia was also reported to be controversial.11 Sodium bicarbonate can potentially increase fluid load, causing hypernatremia and metabolic alkalosis, and should therefore be used with caution in patients with heart failure and CKD because of sodium load. If infused rapidly, it can be metabolized to carbon dioxide, or in those with respiratory insufficiency, can result in acidosis or hyperkalemia. IV sodium bicarbonate may be helpful in patients who require fluid loads.11
Beta2 Agonist: Albuterol inhalation can be considered in nonacute situations to lower potassium. Albuterol stimulates Na+/K+-ATPase, which results in intracellular shift of potas-sium.12,13 Use of albuterol has been shown to decrease serum potassium levels by 0.3 to 0.6 mEq/L within 30 minutes; the decrease lasts for at least 2 hours. Doses from 10 to 20 mg utilized in hyperkalemia are much higher than those used in management of acute bronchospasm. Due to the high doses used and the potential beta1-receptor stimulation, patients may experience tachycardia. Unfortunately, not all patients respond to therapy because of concurrent use of nonselective beta-blockers. The mechanism of resistance to treatment is unknown; there-fore, albuterol should not be used as monotherapy in urgent hyperkalemia.12,14
Diuretics: Following the use of methods to shift potassium into cells, strategies should then be undertaken to eliminate excess potassium. In patients with adequate kidney function, loop diuretics (e.g., furosemide and bumetanide) in combination with thiazide diuretics can be used for the excretion of potassium. Onset of action is 15 to 60 minutes. However, it should be noted that although diuretic-induced volume depletion can lead to decreased distal nephron flow and reduced potassium excretion, volume-expanded patients will benefit from diuresis.12
Sodium Polystyrene Sulfonate (SPS): SPS was first introduced in the 1950s before the FDA was required to establish drugs as both safe and effective.15 SPS is a cation-exchange polymer that exchanges sodium for potassium, in addition to other cations such as calcium, ammonium, and magnesium. The sulfonate groups on SPS will be occupied by hydrogen ions in the presence of acid, thereby reducing its therapeutic efficacy. SPS is sometimes poorly tolerated and produces unpredictable reductions in potassium. SPS also contains a considerable amount of sodium content and should be used cautiously in patients with concomitant conditions such as congestive heart failure, edema, and severe hypertension. It is most effective when it is in the colon, where the pH level is higher than in the upper GI tract. Therefore, it may be given as 15 to 30 g by mouth with cathartics (most commonly sorbitol) or as an enema. The rectal dose is 30 to 50 g but has been found to be less effective compared to an equivalent dose administered orally. SPS does not work as quickly as alternative treatment options; onset of action is >2 hours. The degree to which potassium is reduced and its onset of action are also variable.16
In 2011, the FDA’s Center for Drug Evaluation and Research (CDER) approved safety labeling changes for SPS to include additional warnings.17 SPS may rarely be associated with fatal colonic necrosis and other serious GI adverse events, which are believed to be related to administration with sorbitol. Therefore, it is not recommended to be given with sorbitol (SPS is now available as sorbitol-free powder).11,14 SPS should not be used in patients who do not have normal bowel function, including postoperative patients who have not had a bowel movement after surgery and in patients at risk for developing constipation or impaction. If patients develop constipation, use of SPS should be discontinued, and repeated doses should not be given to those who have not passed a bowel movement.17
Dialysis: Hemodialysis is the method of choice for removal of potassium when pharmacologic therapies fail to adequately lower and eliminate potassium. Within 60 minutes, potassium can decrease by >1 mEq/L and by a total of 2 mEq/L within 180 minutes.12
New Treatment Options
Management of hyperkalemia had remained unchanged until the approval of a new drug in 2015. Patiromer is an option for outpatient management of chronic hyperkalemia in those patients with CKD and on RAAS inhibitors who would benefit from continuation of therapy due to comorbid conditions such as diabetes mellitus and heart failure.18,19 Another agent is also currently undergoing FDA review.
Patiromer (Veltassa): On October 21, 2015, the FDA approved patiromer for the treatment of hyperkalemia.18 Patiromer is a powder for suspension in water for oral administration. The active ingredient is patiromer sorbitex calcium, which contains patiromer, a nonabsorbed potassium-binding polymer with a calcium-sorbitol counterion. By binding potassium in the lumen of the GI tract, patiromer is able to increase fecal potassium excretion.18,19
Patiromer is not for life-threatening hyperkalemia. It is considered an option for patients with CKD and diabetic patients with a serum potassium >5 mEq/L who would benefit from treatment with an ACE inhibitor, angiotensin receptor blocker (ARB), or aldosterone inhibitor.19
Patiromer is recommended to be initiated at 8.4 g once daily (TABLE 1).19 The dose can be adjusted based upon serum potassium levels, with a maximum dose of 25.2 g once daily. The dose can be increased based on serum potassium levels at 1-week or longer intervals, in increments of 8.4 g. Doses should be prepared immediately prior to administration. Patients should measure one-third cup of water. Half of the water should be placed in a glass, then the patiromer packet added and the mixture stirred. The remaining water should be added and the mixture stirred again thoroughly. The powder will not dissolve and the mixture will appear cloudy. More water can be added to the mixture for desired consistency. The mixture should be consumed immediately. If powder remains in the glass after drinking, more water should be added, stirred, and consumed immediately. Repeat as needed until the entire dose is administered.19
This medication is commercially available as single-use packets containing 8.4, 16.8, or 25.5 g. It should be stored in the refrigerator at 2°C to 8°C (36°F-46°F). If stored at room temperature (25°C ± 2°C [77°F ± 4°F]), patiromer must be used within 3 months of being taken out of the refrigerator. The product cannot be used after the printed expiration date on the packet.19
The efficacy of patiromer was demonstrated in a two-part, single-blind, randomized withdrawal trial.19,20 Patients with hyperkalemia and CKD on at least one RAAS inhibitor were included in the study. In Part A, patients with baseline serum potassium of 5.1 to <5.5 mEq/L initiated patiromer at 8.4 g, while patients with baseline serum potassium of 5.5 to 6.5 mEq/L started with a dose of 16.8 g. The primary endpoint was change in serum potassium from baseline to week 4. The patient group with lower baseline potassium showed a decrease of -0.65 + 0.05 (95% CI, -0.74 to -0.55) in serum potassium, while the higher baseline potassium group showed an even larger decrease in serum potassium of -1.23 + 0.04 (95% CI, -1.31 to -1.16).19,20
Part B looked at the placebo-controlled withdrawal phase.19,20 The primary endpoint was change in serum potassium from Part B baseline to the earliest visit when the patient had a serum potassium outside of 3.8 to <5.5 mEq/L or Part B week 4 if the potassium remained in range. Patients who remained on patiromer had no change from baseline, while those switched to placebo had a 0.72 mEq/L (95% CI, 0.46-0.99) increase in serum potassium.19,20
In an open-label study, the effect of treatment with patiromer for up to 52 weeks was evaluated in 304 hyperkalemic patients with CKD and type 2 diabetes on RAAS inhibitors. The treatment effect of patiromer was found to be maintained during long-term therapy.19
When initially approved, patiromer carried a boxed warning because of its ability to bind to other oral medications. On November 27, 2016, the FDA approved a supplemental New Drug Application (sNDA) and removed the boxed warning regarding separation of patiromer and other drugs.21 New recommendations advise patients to take patiromer at least 3 hours before or 3 hours after other oral medications.19,21
Patiromer cannot be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action.19 Use of patiromer should be avoided in patients with severe constipation, bowel obstruction, or impaction since it may be ineffective and worsen GI conditions. Patiromer also has the ability to bind to magnesium in the colon, which can result in hypomagnesemia. It was reported to occur in 5.3% of patients treated. Serum magnesium should be monitored and magnesium supplementation may be needed in patients who develop low serum levels. The most common adverse reactions that occurred in 2% of patients included constipation that generally resolved during the course of treatment, hypomagnesemia, hypokalemia, diarrhea, nausea, abdominal discomfort, and flatulence. Mild-to-moderate hypersensitivity reactions including edema of the lips occurred in 0.3% of patients treated.19
Sodium Zirconium Cyclosilicate (ZS-9): New therapy is on the horizon for management of hyperkalemia. ZS-9 is an insoluble, nonabsorbed compound designed to capture potassium ions. It is a powder formulation mixed with water for oral use. The agent’s site of action is in the GI tract, where it binds potassium and allows for fecal excretion in exchange for sodium and hydrogen counterions. It was found to be specific for potassium in the presence of other ions. The compound is believed to work immediately upon ingestion and continuously as it moves through the GI tract.22,23
ZS-9 has been studied in two phase III, randomized, placebo-controlled, double-blind trials.23,24 HARMONIZE was a two-part trial: open-label 48-hour phase, followed by a 28-day randomized phase. Patients were eligible for the study if two consecutive potassium levels of >5.1 mEq/L were documented. Patients included in the study had prior diagnosis of CKD, heart failure, and diabetes. Patients were also being treated with RAAS inhibitors. The initial endpoint of normalized potassium levels between 3.5 and 5.0 mEq/L at 48 hours was an average decrease of potassium from 5.6 to 4.5 mEq/L achieved by 84% of patients (95% CI, 79%-88%) in 24 hours and 98% (95% CI, 96%-99%) in 48 hours. Those patients who achieved normalized potassium levels in the initial phase were randomized to the second phase in which they received 1 of 3 doses of ZS-9 or placebo for 28 days. The primary endpoint was mean serum potassium during days 8 to 29. All three dose groups showed significantly lower serum potassium levels versus placebo.23,24
Another phase III study looked at ZS-9 in a two-stage, double-blind, international trial.25 Patients were included if they had serum potassium levels of 5.0 to 6.5 mEq/L. Patients included in the study had reduced renal function (estimated glomerular filtration rate [eGFR] <60 mL/min), diabetes, and heart failure. Two-thirds of the patients were receiving RAAS inhibitors at baseline. In the initial phase, patients received either placebo or ZS-9 at doses of 1.25, 2.5, 5 g, or 10 g, 3 times/day with meals for 48 hours. The endpoint for the initial phase was rate of change of potassium in 48 hours. All groups that received ZS-9 saw decreases in potassium within 48 hours. All patients who achieved normal potassium levels in the range of 3.5 to 4.9 mEq/L received either their original ZS-9 dose or placebo for days 3 to 14 for the maintenance phase. All patients receiving ZS-9 for days 3 to 14 were found to have decreased potassium levels. Treatment effect was observed within 1 hour of administration and maintained throughout days 3 to 14.23,25
Trials for ZS-9 are ongoing. After receiving a Complete Response Letter from the FDA in May 2016, which referred to observations from a preapproval manufacturing inspection, AstraZeneca resubmitted its NDA in October 2016. However, ZS-9 is not currently approved in any market.22,26
Role of the Pharmacist
Pharmacists can play a vital role in the management of hyperkalemia. They should be aware of newly approved treatment options and can assist with dosing of the various medications used, as well as assess for drug-inducing hyperkalemia agents. Since management options for hyperkalemia have remained consistent for many years prior to the approval of patiromer, the only comparator of the newly approved agent was SPS. Although the data regarding efficacy were limited for SPS, it became a mainstay treatment option due to lack of alternatives. The 2015 approval of patiromer now adds another option for management of hyperkalemia in the outpatient setting. Neither patiromer nor ZS-9 replace current treatment approaches for hyperkalemia. ZS-9 may be beneficial as suppressive or preventive treatment in those with CKD.
REFERENCES
1. Tran HA. Extreme hyperkalemia. South Med J. 2005;98(7):729-732.
2. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
3. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
4. Fleet JL, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open. 2012;2(6):e002011.
5. Mount DB, Zandi-Nejad K. Disorders of potassium balance. In: Brenner BM, ed. Brenner and Rector’s The Kidney. 8th ed. Philadelphia, PA: WB Saunders; 2008:547.
6. Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40-47.
7. Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3(2):324-330.
8. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182:1631-1635.
9. Soar J, Perkins GD, Abbas G, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac arrest in special circumstances: electrolyte abnormalities, poisoning, drowning, accidental hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution. Resuscitation. 2010;81(10):1400-1433.
10. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S829-S861.
11. Rossignol P, Legrand M, Kosiborod M, et al. Emergency management of severe hyperkalemia guidelines for best practice and opportunities for the future. Pharmacol Res. 2016;113(pt A):585-591.
12. Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008;36(12):3246-3251.
13. Wong SL, Maltz HC. Albuterol for the treatment of hyperkalemia. Ann Pharmacother. 1999;33(1):103-106.14. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554.
15. O’Brien T. New questions on old drug for hyperkalemia. Kidney News. 2010;2(5):1-5.
16. Mistry M, Shea A, Giguère P, Nguyen ML. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother. 2016;50(6):455-462.
17. Kayexalate (sodium polystyrene sulfonate) powder. Safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER). January 2011. www.fda.gov/Safety/MedWatch/SafetyInformation/ucm186845.htm. Accessed January 20, 2017.
18. FDA approves new drug to treat hyperkalemia. FDA news release. October 21, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468546.htm. Accessed October 9, 2016.
19. Veltassa (patiromer for oral suspension) prescribing information. Redwood City, CA: Relypsa Inc; November 2016.
20. Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211-221.
21. Relypsa Inc. FDA approves supplemental new drug application for Veltassa removing boxed warning regarding drug-drug interactions. Press release. November 27, 2016. www.relypsa.com/newsroom/press-releases/112716. Accessed January 21, 2017.
22. AstraZeneca. FDA accepts for review New Drug Application for sodium zirconium cyclosilicate (ZS-9) for the treatment of hyperkalaemia. Press release. October 18, 2016. www.astrazeneca.com/media-centre/press-releases/2016/fda-accepts-for-review-new-drug-application-for-sodium-zirconium-18102016.html. Accessed November 10, 2016.
23. Linder KE, Krawczynski MA, Laskey D. Sodium zirconium cyclosilicate (ZS-9): a novel agent for the treatment of hyperkalemia. Pharmacotherapy. 2016;36(8):923-933.
24. Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223-2233.
25. Packham DK, Rasmussen HS, Lavin P, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222-231.
26. AstraZeneca receives Complete Response Letter from US FDA for sodium zirconium cyclosilicate (ZS-9) for oral suspension for treatment of hyperkalaemia. Press release. May 27, 2016. www.astrazeneca.com/media-centre/press-releases/2016/astrazeneca-receives-complete-response-letter-from-us-fda-for-sodium-zirconium-cyclosilicate-zs-9-for-oral-suspension-for-treatment-of-hyperkalaemia-27052016.html. Accessed November 20, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.






