US Pharm. 2017;42(3):33-37.
ABSTRACT: Gout is one of the most common rheumatologic arthritic diseases in the United States. Preventing future gout attacks requires lowering serum urate levels to promote dissolution of urate crystals, which is accomplished by reducing the production of serum uric acid or promoting its excretion. Xanthene oxidase inhibitors (XOIs), including allopurinol and febuxostat, are first-line agents for the prevention of acute attacks. Probenecid, a uricosuric agent, is an appropriate adjunctive therapy or second-line agent when XOIs are contraindicated or poorly tolerated. Recently, several third-line agents (e.g., pegloticase, lesinurad) have become available for the treatment of refractory gout.
Gout is a rheumatologic arthritic disease that manifests itself after prolonged exposure to excessive levels of serum uric acid (hyperuricemia). Eventually, urate crystals precipitate and deposit into joint spaces. This process initiates an inflammatory reaction, which results in severe pain around the affected joints. Gout pain often presents in the distal joints. Pharmacologic goals include minimizing the inflammation and pain of an acute gout attack and preventing future gout flares. Initial urate-lowering therapy (ULT) usually consists of diet modification. Pharmacologic therapy lowers serum urate by either reducing the production or increasing the excretion of serum urate.1
ETIOLOGY
Gout is characterized by intense pain and inflammation resulting from the buildup of monosodium urate (MSU) monohydrate crystals in the synovial joints. MSU crystals materialize after prolonged periods of hyperuricemia (serum uric acid levels >6.8 mg/dL).2 Most hyperuricemia patients never experience a flare-up of gout. When MSU crystals deposit in the soft tissue of the synovial joints, interleukin-1 (IL-1) and prostaglandins are activated. This initiates an inflammatory response leading to intense arthritic pain.1,3 Patients generally experience gout pain in the peripheral joints, most often the great toe, due to increased accumulation of urate crystals and lower body temperature in these joints.4 However, joints of the ankle, knee, finger, wrist, and elbow can also be affected.
During an acute gout attack, joints may be swollen or red. Systemic symptoms, such as fever, may also be present.1 Initial episodes usually resolve spontaneously within 1 to 2 weeks.5 However, the patient is at risk of inflammation, joint destruction, and future gout attacks. As hyperuricemia progresses, the interval between acute gout attacks decreases. Prolonged inflammation can progress to chronic arthritis in one or more joints. Tophi (nodules manifested by MSU crystals in the joint, cartilage, and bone) are a common clinical feature of gout and are important in the diagnosis of the disease.1
EPIDEMIOLOGY
One of the most common causes of inflammatory arthritis in the United States is gout. Approximately 8 million Americans are affected by gout and that number continues to rise.6 Conditions such as hypertension, metabolic syndrome, type 2 diabetes mellitus, heart failure, organ transplant, obesity, and chronic kidney disease (CKD) promote an increased incidence of hyperuricemia and gout.1,7 Additionally, medications may promote elevated uric acid levels. Diuretics are particularly problematic due to potential hypovolemia and decreased renal elimination of uric acid.8
A variety of risk factors are associated with gout including age, hypertension, overweight or obesity, diuretics, and diet.9,10 Mutations in genes such as urate-transporter genes SLC17A1 and ABCG2 are also associated with an elevated risk of gout.11 Hyperuricemia potentially leads to other disease states including nephrolithiasis (kidney stones), renal failure, and cardiovascular disease.1,5,12 Although rare, chronic interstitial nephropathy may develop due to the accumulation of MSU crystals in the medulla of the kidney.1
DIAGNOSIS
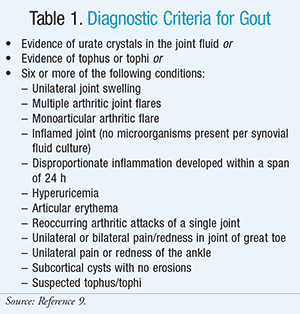
Hyperuricemia alone is not sufficient for the diagnosis of gout. Observation of MSU crystals or the presence of tophi is required for diagnosis and initiation of ULT. A diagnosis is possible if a combination of several key clinical signs or laboratory values are present including arthritic flare in a single joint, excessive inflammation within a 24-hour period, joint redness, pain in the great toe, inflammation without infection in the synovial fluid, or subcortical cyst with no erosions (Table 1).9
NONPHARMACOLOGIC TREATMENT
The American College of Rheumatology (ACR) recommends diet modification for all gout patients. Uric acid is a byproduct of the metabolism of purines.1 Meat and seafood should be consumed in moderation.3,13 Foods high in purine content, such as organ meats (e.g., kidneys, liver) should be avoided. Fructose is the only carbohydrate associated with elevated serum uric acid levels, and is believed to increase the synthesis or metabolism of purines. Studies have shown that consumption of fructose (e.g., high fructose corn syrup food and beverages) is associated with elevated serum urate levels and should be avoided.3,14 Alcohol of all types should also be avoided because it elevates serum urate levels by increasing the production and reducing the elimination of uric acid.15
Increased consumption of low-fat dairy products and vegetables are associated with lower serum uric acid levels.3,13,16 Additionally, increased vegetable intake is associated with a reduced risk of nephrolithiasis.16 Recent studies estimate that dietary changes alone account for serum uric acid reductions of up to 18%.17 However, for patients with serum uric acid levels >7 mg/dL, dietary adjustments alone are not sufficient to achieve target serum uric acid goals of <6 mg/dL.3 In these patients, pharmacologic treatment is recommended.
PHARMACOLOGIC TREATMENT
Management of gout falls into two categories: treatment of acute gout attacks and prophylaxis of gout flares. The goal of treating acute attacks is to resolve arthritic pain and inflammation. Typically, oral or injectable nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or oral colchicine are prescribed.10,13
Prophylactic treatment of gout flare-ups consists of lowering serum urate levels by reducing the production of uric acid or increasing the excretion of urate from the body. Accumulation of serum uric acid is often due to diminished excretion from the kidneys and not over-production. ULT is indicated when a history of gout is present plus at least one of the following: single tophus or tophi, a history of nephrolithiasis, or at least two gout flares per year. Initiating ULT is also indicated with hyperuricemia (serum urate levels >6.8 mg/dL) and CKD stage 2 or higher, including end-stage renal disease. ULT should be initiated during an acute gout attack along with an anti-inflammatory regimen.3
A target serum urate level of <6 mg/dL promotes gradual dissolution of MSU crystals. Some patients may require serum urate levels <5 mg/dL to see resolution of symptoms.10,13 Serum urate should be monitored every 2 to 5 weeks during initiation and titration of ULT. Once the serum urate level has reached goal, monitoring should continue every 6 months with ongoing treatment.3
Paradoxically, the onset of treatment to decrease serum uric acid levels is associated with acute gout flare. While the mechanism behind this phenomenon is unknown, it has been hypothesized that the initial lowering of serum urate may activate previously precipitated crystals.18 This may be why an estimated 56% of gout patients are nonadherent with ULT within the first year of therapy.19 After 1 year of therapy, the risk of ULT-induced acute flares tends to abate with the decreased serum urate levels. ULT-induced acute gout flares are extremely rare after 5 years of therapy.20
Xanthene Oxidase Inhibitors (XOIs)
Xanthene oxidase is an enzyme that converts hypoxanthine into xanthene and then xanthene into uric acid during purine metabolism. Suppression of this enzyme is the target of the XOIs and is responsible for reducing uric acid production.1 Minimizing uric acid synthesis with XOIs is the preferred mechanism for lowering serum urate levels. Allopurinol and febuxostat are currently the only two FDA-approved XOIs. If one fails to significantly lower uric acid levels or is poorly tolerated, then the other is considered an appropriate selection.3 Febuxostat has one advantage over allopurinol. For patients with mild-to-moderate renal impairment, febuxostat dosing does not need to be adjusted.21
The European League Against Rheumatism (EULAR) guidelines differ from the ACR guidelines in the recommendation of utilizing allopurinol as the first-line XOI agent due to the cost and effectiveness of both agents. In addition, they recommend adjusting the dosing of allopurinol with respect to the creatinine clearance (CrCl) in patients with renal failure due to the increased risk of serious cutaneous adverse reactions and the availability of febuxostat as an alternative.13
Allopurinol (Aloprim, Zyloprim): Allopurinol should be initiated at 100 mg daily to minimize the risk of ULT-induced gout flare and the threat of allopurinol hypersensitivity. It should be titrated by 50 to 100 mg every 2 to 5 weeks to the dose required to achieve goal serum urate levels.13 The maximum daily dose of allopurinol is 800 mg.3
The ACR guidelines recommend that patients with CKD stage 4 and higher or a CrCl <30 mL/min should be initiated at a reduced allopurinol dose of 50 mg and titrated to goal serum urate levels. These patients can be titrated above a 300-mg daily dose, but must be monitored for signs of toxicity including pruritus, rash, and elevated hepatic enzymes.3,22 Contrary to ACR guidelines, the FDA recommends standardized dosage adjustments based on renal function. Patients with a CrCl between 10 and 20 mL/min have a maximum daily dose of 200 mg, and those with a CrCl between 3 and 10 mL/min have a maximum daily dose of 100 mg. Patients with CrCl of <3 mL/min should receive doses of 100 mg in extended intervals, more than 24 hours apart. Clinically significant drug interactions include azathioprine, mercaptopurine, cyclophosphamide, captopril, enalapril, and warfarin. Allopurinol is contraindicated with concurrent use of didanosine because of potential increases in serum concentrations of didanosine.1
Allopurinol is associated with a severe hypersensitivity that manifests as eosinophilia, vasculitis, hepatitis, Stevens-Johnson syndrome, or toxic epidermal necrosis.3,23 Reactions usually occur within the first few months of initiation. An estimate of 1 in 1,000 patients experiences a hyper-sensitivity reaction.23 The mortality rate of allopurinol hypersensitivity reactions can be up to 25%.24 Patients who express the HLA-B*5801 gene are also at a much higher risk of hypersensitivity.23 Some ethnic populations express this gene more frequently. HLA-B*5801 screening should be considered in patients of Korean descent with stage 3 CKD or higher. Additionally, testing should be considered in all patients of Han Chinese or Thai descent regardless of kidney function.3,10 For HLA-B*5801–positive patients, allopurinol is not recommended.2,22 Recent studies suggest that additional potential risk factors for hypersensitivity include concurrent use of certain medi-cations (e.g., thiazide diuretics), renal impairment, and the initiation of allopurinol at higher starting doses.23,25
Febuxostat (Uloric): The starting dose of febuxostat is 40 mg/day and may be titrated to 80 mg/day. While the FDA-approved maximum daily dose is 80 mg per day,10,26 doses of 120 mg/day are approved outside of the U.S.13 However, the ACR guidelines recommend doses of 120 mg/day for patients who do not achieve goal serum urate levels.3 Dosage adjustments are generally not needed in patients with mild-to-moderate renal impairment, although caution should be exercised in patients with severe impairment (CrCl <30 mL/min). The most common adverse reactions reported included rash and nausea. Cases of hepatic failure have been reported, necessitating monitoring of hepatic enzymes upon initiation of therapy. Treatment should be discon-tinued if alanine aminotransferase (ALT) increases by more than three times the normal level or if serum total bilirubin increases to twice the normal level. Since febuxostat increases serum concentration of mercaptopurine and azathioprine, concurrent use is contraindicated.26
Uricosuric Agents
Uric acid is excreted through both the kidneys (67%) and the gastrointestinal (GI) tract (33%) with the kidneys reabsorbing 90% of the filtered uric acid. Uricosuric agents such as probenecid inhibit this reabsorption.1 These agents should be initiated only after the maximum daily dosage of XOI therapy has been reached, the patient cannot tolerate XOI adverse effects, or XOI use is contraindicated.13
Probenecid: Probenecid is considered a first-line uricosuric agent.3 It should be initiated at a dose of 250 mg orally twice daily for 1 week, then increased to 500 mg twice daily, with additional titration of 500 mg monthly to a maximum daily dose of 2,000 mg.10,27 Probenecid may be used in combination with an XOI or as monotherapy in refractory gout.3,13 It is contraindicated during an acute gout attack and treatment should be delayed until resolution of symptoms. Salicylates such as bismuth subsalicylate reduce the uricosuric effect. Therefore, concurrent use is contraindicated.27 Common adverse effects include rash, hypersensitivity, and GI discom-fort.1 Long-term therapy has an association with increased incidence of nephrolithiasis and is not recommended in patients with a history of nephrolithiasis or severe renal impairment. Risk of nephrolithiasis is reduced by increasing consumption of water or through urinary alkalization with potassium citrate or sodium bicarbonate.27 Urine urate levels need to be monitored at baseline for all patients.3 Use with caution in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency and peptic ulcer disease.27
Other Agents: Some studies have shown that medications such as fenofibrate, losartan, and calcium channel blockers also have uricosuric properties. Fenofibrate, a triglyceride-lowering medication, reduces uric acid levels by 20%.28 Losartan, an angiotensin receptor blocker (ARB) increases the excretion of uric acid and also alkalinizes urine.29 It should be noted that losartan is the only ARB that has urate-lowering properties.30 Calcium channel blockers are also associated with increased uric acid excretion.30 Although these agents are not FDA-approved for the treatment of gout, they may be beneficial in treating comorbidities due to their uricosuric effect.13
Third-Line Agents
Pegloticase (Krystexxa): Pegloticase is an enzyme that converts uric acid to allantoin, a renally eliminated purine metabolite, thus lowering serum uric acid levels. Pegloticase is administered biweekly as an 8 mg IV infusion in 250 mL of normal or half-normal saline and is administered over no less than 120 minutes, minimizing potential adverse reactions such as itching, redness, shortness of breath, and hives.31 One study found that 26% of patients experienced some form of infusion reaction including severe anaphy-laxis.32 Anti-pegloticase antibodies may form and are associated with an increased risk of infusion reactions.32 It is recommended that patients be pretreated with an anti-histamine and corticosteroid. Patients should be monitored for anaphylaxis for about 1 hour.31
Pegloticase is recommended for use in patients with clinically severe crystal-proven gout that has failed to reach the target uric acid goal at the maximum dose of XOI and uricosuric agents, including a combination of the drugs.3,13 It is contraindicated with the use of other ULT and in patients with G6PD deficiency due to an increased risk of hemolysis and methemoglobinemia. African American, southern Asian, and Mediterranean patients are at higher risk of G6PD deficiency and should be screened prior to treatment.31
Rasburicase (Elitek): Rasburicase is another urate oxidase enzyme similar to pegloticase. It is currently indicated for hyperuricemia in patients receiving cancer treatment resulting in tumor lysis syndrome.33 Rasburicase is not indicated for the treatment of gout due to limited studies. However, it may have the potential to treat gout flares based on its ability to reduce serum uric acid.
Lesinurad (Zurampic): Lesinurad was approved by the FDA in December 2015 for the treatment of refractory hyperuricemia. It is only indicated as an adjunctive therapy in combination with an XOI. It carries a black box warning that, when used as monotherapy, it can increase the risk of acute renal failure. Therefore, lesinurad should not be used as a monotherapy.34
Lesinurad inhibits transporter proteins in the kidney blocking the reabsorption of uric acid. It comes as a 200-mg tablet and should be taken in the morning at the same time as the XOI. This agent is not recommended for patients with a CrCl <45 mL/min. It is contraindicated in patients with a CrCl of <30 mL/min or on dialysis. Patients with a CrCl of <60 mL/min should be monitored frequently for adverse effects. If serum creatinine levels increase to two times the normal limit, discontinuation is recommended. Studies have shown an increased incidence of severe cardiac events, such as myocardial infarction and stroke, although no direct relationship has been established. Lesinurad has the potential to lower the efficacy of hormonal contraceptives.34
PREVENTION OF ULT-INDUCED GOUT FLARE
Concurrent anti-inflammatory prophylaxis is recommended for all patients during the initiation of ULT to lower the risk of ULT-induced gout flare. Anti-inflammatory prophylaxis usually consists of an oral NSAID, colchicine, or a low-dose corticosteroid added to ULT. If a patient has a gout flare while taking ULT, therapy should be continued uninterrupted.35
Colchicine is a first-line option and should be taken orally at a dose of 0.6 mg once or twice daily.35 Contraindications to colchicine include CKD and chronic hepatitis.36 Low-dose NSAIDs, such as naproxen 250 mg taken twice daily, are another option for ULT-induced flare prophylaxis.35 Contraindications to NSAIDs include hypertension, cardiovascular disease, CKD, and gastroesophageal disease.36 Proton pump inhibitors should be considered for patients at risk of peptic ulcer disease. The most common adverse effects of these two drugs include upper respiratory infections, musculoskeletal problems, and diarrhea.37
While oral corticosteroid use is not preferred, it may be appropriate in patients for whom NSAID or colchicine use is contraindicated or ineffective. Only low-dose steroids (equivalent to prednisone 10 mg per day or less) are considered appropriate.35 The efficacy of low-dose corticosteroid use is not conclusive. Additionally, the adverse-effect profile of corticosteroid use needs to be considered in determining the appropriateness of therapy.
Prophylaxis should continue for a minimum of 6 months.13 For patients without manifestations of tophi, 3 months of therapy is recommended after achieving target serum urate levels. For patients with detectable but resolved tophi, 6 months of treatment is recommended after achieving target serum urate levels.35 Patients who have had gout longer will likely require a longer duration of anti-inflammatory prophylaxis.38
Several studies have concluded that inhibition of IL-1 is a promising mechanism for the anti-inflammatory prophylaxis of ULT. Research has shown that rilonacept, anakinra, and canakinumab are agents that are potentially effective as off-label treatment for ULT-induced gout flare.5,39,40 The most common adverse reactions of these agents are injection-site reactions. However, they were otherwise well tolerated for short-term use. Increased risk of acquired infection is also a concern with IL-1 suppression and patients should be screened prior to initiation.5 The EULAR guidelines recommend that for patients with contraindications to colchicine, corticosteroids, and NSAIDs, an IL-1 blocker should be prescribed.13
PHARMACIST INVOLVEMENT
Pharmacists are encouraged to monitor for and address the use of medications that cause elevated serum urate levels. These drugs include thiazide and loop diuretics, niacin, and calcineurin inhibitors such as tacrolimus and cyclosporine.3,8 Since most pharmacotherapies, with the exception of febuxostat, should be dosed based on renal function, pharmacists should be aware of the patient’s kidney function and recommend dosage adjustments when appropriate. Pharmacists should ensure the appropriate titration of ULT and proper use of gout flare prophylaxis to minimize the risk of ULT-induced gout flare and increase patient adherence. Monitoring for common drug interactions such as concurrent use of probenecid and salicylates is important to patient safety. Proper patient counseling should include the benefits of diet modification (see Patient Resources).

CONCLUSION
Dietary modification is typically the initial treatment for patients with gout. For patients who do not reach target serum uric acid goals with diet changes alone, pharmacologic therapy is required to reduce the production or increase the excretion of serum uric acid. There are first-, second-, and third-line therapies available to reach the desired serum uric acid levels. Rapidly lowering serum urate may initiate episodes of acute gout flare. ULT should be titrated and accompanied by gout flare prophylaxis upon initiation.
REFERENCES
1. Wilson L, Saseen JJ. Gouty arthritis: a review of acute management and prevention. Pharmacotherapy. 2016;36(8):906-922.
2. Neogi T. Gout. N Engl J Med. 2011;364(5):443-452.
3. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431-1446.
4. Kippen I, Klinenberg JR, Weinberger A, Wilcox WR. Factors affecting urate solubility in vitro. Ann Rheum Dis. 1974;33(4):313-317.
5. Schumacher HR Jr, Evans RR, Saag KG, et al. Rilonacept (interleukin-1 trap) for prevention of gout flares during initiation of uric acid-lowering therapy: results from a phase III randomized, double-blind, placebo-controlled, confirmatory efficacy study. Arthritis Care Res. 2012;64(10):1462-1470.
6. Zhu Y, Pandya BJ, Choi HK, et al. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
7. McAdams-Demarco MA, Maynard JW, Baer AN, Coresh J. Hypertension and the risk of incident gout in population-based study: the atherosclerosis risk in communities cohort. J Clin Hypertens. 2012;14(10):675-679.
8. Hunter DJ, York M, Chaisson CE, et al. Recent diuretic use and the risk of recurrent gout attacks: the online case-crossover gout study. J Rheumatol. 2006;33(7):1341-1345.
9. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
10. Qaseem A, Harris RP, Forciea MA. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(1):58-68.
11. Reginato A, Mout DB, Yang I, Choi HK. The genetics of hyperuricemia and gout. Nat Rev Rheumatol. 2012;8(10):610-621.
12. Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005;118(8):816-826.
13. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29-42.
14. Choi HK, Curhan G. Soft drinks, fructose consumption and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
15. Neogi T, Chen C, Niu J, et al. Alcohol quantity and type on risk of recurrent gout attacks: an internet-based case-crossover study. Am J Med. 2014;127(4):311-318.
16. Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52(1):283-289.
17. Dessein PH, Shipton EA, Stanwix AE, et al. Beneficial effects of weight loss associated with moderate calorie/ carbohydrate restriction and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis. 2000;50(7):539-543.
18. Becker MA, MacDonald PH, Hunt BJ, et al. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):585-591.
19. Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11(2):R46.
20. Schumacher HR Jr, Becker MA, Lloyd E, et al. Febuxostat in the treatment
of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology. 2009;48(2):188-194.
21. Grassi D, Pontremoli R, Bocale R, et al. Therapeutic approaches to chronic hyperuricemia and gout. High Blood Press Cardiovasc Prev. 2014;21(4):243-250.
22. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
23. Ramasamy S, Korb-Wells CS, Kannangara DR, et al. Allopurinol hyper-sensitivity: a systematic review of all published cases, 1950-2012. Drug Safety. 2013;36(10):953-980.
24. Hershfield MS, Callaghan JT, Tassaneeyakul W, et al. Clinical Pharmaco-genetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013;92(2):153-158.
25. Markel A. Allopurinol-induced DRESS syndrome. Isr Med Assoc J. 2005;7(10):
656-660.
26. Uloric (febuxostat) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; March 2013.
27. Probenecid package insert. New Castle, DE: Marlex Pharmaceuticals, Inc; May 2013.
28. De la Serna G, Cadarso C. Fenofibrate decreases plasma fibrinogen, improves lipid profile, and reduces uricemia. Clin Pharmacol Ther. 1999;66(2):166-172.
29. Shahinfar S, Simpson RL, Carides AD, et al. Safety of losartan in hypertensive patients with thiazide-induced hyperuricemia. Kidney Int. 1999;56(99):1879-1885.
30. Choi HK, Soriano LC, Zhang Y, Rodríguez LA, et al. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case control study. BMJ. 2012;344:d8190.
31. Krystexxa (pegloticase) package insert. Glendale, WI: Crealta Pharmaceuticals LLC; December 2014.
32. Sundy JS, Baraf HS, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment. JAMA. 2011;306(7):711-720.
33. Vadhan-Raj S, Fayad LE, Fanale MA, et al. A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2012;23(6):1640-1645.
34. Zurampic (lesinurad) package insert. Wilmington, DE: Astra Zeneca Pharmaceuticals LP; 2015.
35. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447-1461.
36. Keenan RT, O’Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124(2):155-163.
37. Wortman RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Therapeutics. 2010;32(14):2386-2397.
38. Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis. 2007;66(8):1056-1058.
39. Ottaviani S, Moltó A, Ea HK, et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther. 2013;15(5):R123.40. Schlesinger N, Mysler E, Lin HY, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomized study. Ann Rheum Dis. 2011;70(7):1264-1271.
To comment on this article, contact rdavidson@uspharmacist.com.






