ABSTRACT: Gastrointestinal bleeds (GIBs) are a significant source of hospitalizations in the United States. GIBs are categorized into two types: upper GI bleeds and lower GI bleeds. Each type can present with different hallmark presentations and require different management. Upper GIB is dependent on endoscopic therapy and may benefit from various pharmacologic treatments in specific scenarios, such as proton pump inhibitors and macrolides. Management of lower GIB is focused on colonoscopy and mechanically treating bleeds, with little pharmacological intervention. Pharmacists can provide effective service to these patients at multiple points of patient care.
Gastrointestinal bleeding (GIB) is a relatively common medical issue, causing a significant portion of morbidity, hospitalizations, and even mortality annually in the United States. The raw estimated rate of hospitalization secondary to any type of GIB in the U.S. is estimated to be about 375 per 100,000 per year.1-3 For acute GIB events, some studies identify a 30-day mortality rate as high as 14%, while others report a range between 6% and 10% per year, with rates increasing in patients with advancing age and a higher number of associated underlying comorbidities.4-7 It is estimated that more than $2.5 billion is spent annually to care for inpatient management of GIB.8
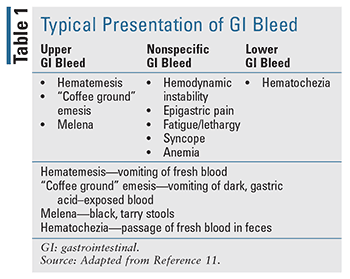
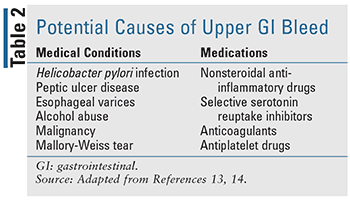
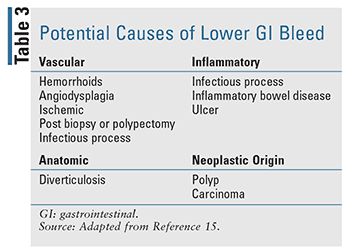
Given the nature of GIB, it is important to differentiate the disease state, as location of the bleed can dictate both presentation and treatment (TABLES 1-3). Historically, distinction of upper GIB (UGIB)and lower GIB (LGIB) was based on the location of bleeding in relation to the ligament of Treitz.9,10 With this definition, bleeding proximal to the ligament of Treitz is categorized as an UGIB, while bleeding distal to the ligament of Treitz is categorized as a LGIB. While other definitions do exist in some literature, this definition is generally established in most practice.11,12
Initial Presentation and Risk Stratification
At presentation, workup of any suspected GIB is initially similar, wherever the origin of the bleed. Immediate evaluation should focus on the patient’s hemodynamic stability. This priority has been well established in other hemorrhagic models and remains true in cases of GIB.16-18 Physical assessment of blood pressure, heart rate, and orthostatic status should be performed, and resuscitation provided as appropriate. This resuscitation should begin with intravenous fluids but may also require transfusions of packed red blood cells.
Additional assessment of patients presenting with GIB should include a focused patient history aimed at identifying the nature, duration, and potential source of the bleeding, as well as laboratory testing (CBC, BMP, coagulation studies).17,18 When evaluating these patients from a pharmacist perspective, it is important to note any current or recent medication use that may increase the risk of bleeding, including agents such as nonsteroidal anti-inflammatory drugs, antiplatelet agents, and/or anticoagulants. Early identification of these factors may help expedite both diagnosis and appropriate treatment response.
Risk assessment and stratification is clinically useful to help distinguish patients at high and low risk of adverse outcomes.17,18 In stratifying patients into evaluated risk groups, initial informed decision-making can be made. Specifically, stratification may provide insight into factors such as timing of endoscopy or colonoscopy, necessary level of care, and timing of discharge. Various established tools are available to aid in the assessment of individual risk.
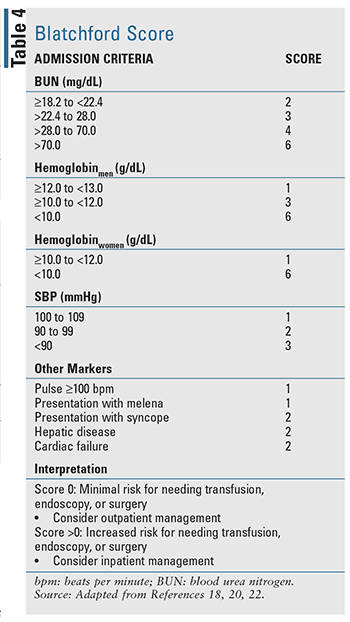
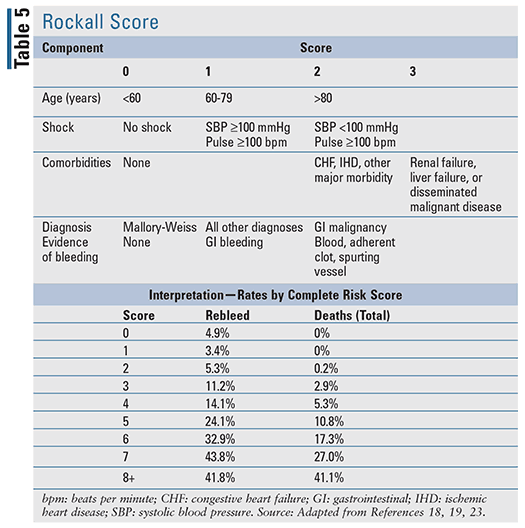
For patients with acute UGIB, there are several clinical prediction tools that have been developed, including two well-evaluated tools cited in evidence-based guidelines: the Blatchford score (TABLE 4) and the Rockall score (TABLE 5).17-20 The Rockall score utilizes clinical data immediately at presentation.19 Factors assessing the severity of the bleed, such as systolic blood pressure and heart rate, are assessed alongside patient characteristics, such as age and comorbid conditions, resulting in a tool to assess prognostic outcomes and potentially mortality. Similarly, the Blatchford score uses both clinical and laboratory data to assist in predicting risk of intervention and mortality.20 For acute LGIB, the available prognostic rules to assess risk are limited and less validated when compared with UGIB.22
Upper GI Bleed Pharmacotherapy
Acid Suppression
Proton pump inhibitors (PPIs) are the drug class most associated with treatment of UGIB. In an UGIB patient, gastric acid can inhibit platelet aggregation and weaken potential coagulation, leading to an impairment in bleeding cessation. To inhibit this process, inhibition of gastric acid secretion intended to raise stomach pH to 6 or higher can help stabilize clots and improve clinical outcomes.24
PPIs are unique in that they have potential application both pre- and postendoscopy. In the setting of pre-endoscopy, guidelines do not form consensus on their use, but they may decrease proportion of patients with high risk of stigmata of hemorrhage at the time of the procedure.25 Despite potential benefit in this population, evidence suggests PPI use does not affect rebleeding, necessary surgery, or mortality, leading to some clinical controversy regarding their application. However, patients for whom endoscopy cannot be performed or will be delayed have demonstrated a potential decrease in risk of rebleeding through utilization of PPI. Evidence suggests PPIs decrease incidence of high-risk stigmata of hemorrhage at time of endoscopy but do not affect rebleeding, necessary surgery, or mortality.17,26 Postendoscopic therapy, PPIs can be used both acutely and chronically.17
Continuous Infusion Versus Intermittent PPI
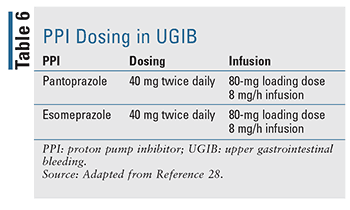
Appropriate dosing and administration of PPI have been evaluated closely in recent years (TABLE 6). A bolus IV dose followed by infusion was long the normal course of therapy and remains the treatment of choice in currently available guidelines, but data suggest that twice-daily IV bolus dosing is noninferior in outcomes of rebleeding, mortality, and length of hospital stay.17,27 Recent literature has demonstrated that intermittent PPI use was noninferior and offers a clear benefit in drug dose, cost, and resource utilization. The authors suggest the that guidelines be updated to recommend intermittent PPI therapy in endoscopically treated GIB.27 In practice, many institutions now favor intermittent dosing in order to decrease resource use and overall cost.
Intravenous PPI Versus Intravenous H2 Antagonists
Considering H2 antagonists (H2As) are used with similar intent in other disease states, their use in UGIB has been investigated alongside PPIs. A 2015 meta-analysis evaluating the two classes in the setting of UGIB found that while difference in mortality was found to be nonsignificant, outcomes such as recurrent bleeding rate and receiving surgery rate displayed clear benefit in PPIs. This deficit is likely due to H2As’ inferiority at maintaining gastric pH over 6.0, the approximate pH where coagulation processes can function.29
Macrolides
In effort to improve visualization during endoscopy and thus decrease need for repeat endoscopy, IV erythromycin, an antimicrobial macrolide that also acts as a prokinetic agent, is commonly utilized. A single dose of 250 mg or 3 mg/kg is generally well tolerated.17,30 A 2016 meta-analysis concluded erythromycin use prior to endoscopy significantly decreased the need for second-look endoscopy and length of hospital stay, and thus it carries a recommendation to be used in the European Society of Gastrointestinal Endoscopy guidelines.30,31
It is fair to wonder if possible benefit is a class effect or specific to erythromycin. Azithromycin, a generally more commonly utilized macrolide, has been evaluated and appears to be a noninferior alternative to erythromycin. Azithromycin may carry some logistical advantages, such as not requiring reconstitution and generally greater availability due to its use in empiric pneumonia treatment.32
H pylori Treatment
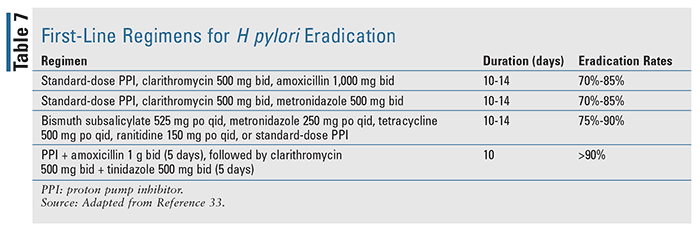
Patients diagnosed with bleeding associated with Helicobacter pylori infection should be treated via guideline-based eradication therapy, with a goal of complete infection resolution.17 Available literature indicates that eradication of H pylori is significantly more effective than antisecretory treatment alone in patients with UGIB. The first-line regimens for H pylori eradication are detailed in TABLE 7 based on the American College of Gastroenterology’s guidelines on the management of H pylori infection.33 Once the infection is resolved, this group of patients typically does not need to receive maintenance antisecretory therapy unless they require nonsteroidal anti-inflammatory drugs or antithrombotic therapy, with which long-term antisecretory therapy is necessary, as their use can significantly increase the risk of rebleeding.17,18
Lower GI Bleed
Treatment Overview
After the initial assessment and risk stratification and once the patient is hemodynamically stable, colonoscopy preceded by colon cleansing is the initial diagnostic procedure for most patients presenting with a LGIB.21 Management of LGIB mostly includes nonpharmacologic interventions; however, there are still opportunities for pharmacists to be involved in patient care.
PPI Application in LGIB
Unlike UGIB, available literature evaluating PPI use in LGIB does not show benefit. Trials evaluating UGIB and LGIB have demonstrated that while PPIs do not demonstrate improved outcomes in LGIB, they may actually increase risk of LGIB.34 Theorized mechanisms for this increased risk often focus on possible changes in microbiota, and thus may potentiate possible risk from NSAIDs. These outcomes have proved controversial; however, they offer evidence that patients with LGIB do not benefit from PPI therapy. This potential risk should be carefully assessed in patients, particularly those at risk for UGIB, as careful risk versus benefit may need to be assessed.34
Pharmacist’s Role
Pharmacists of many specialties are in a unique position to assist in the treatment and management of GIB patients. Outpatient pharmacists are in a prime position to proactively watch for potential risk factors in patients, including high-dose NSAIDs and anticoagulants, and reduce the chance of GIB at the front line. Additionally, they can provide important counseling and dose optimization for patients both prior to and subsequent to a GIB diagnosis. Similarly, inpatient clinical pharmacists can also have a large role in GIB treatment. They may be in a position to assist in multiple areas during the initial evaluation and management. Initial medication reconciliation may reveal details relevant to diagnosis and treatment of patients, such as identifying outpatient NSAID or anticoagulant use. Pharmacists can also provide recommendations to the healthcare team regarding which medications may provide benefit to each GIB case.
REFERENCES
1. El-Tawil A. Trends on gastrointestinal bleeding and mortality: where are we standing? World J Gastroenterol. 2012;18(11):1154-1158.
2. Zhao Y, Encinosa W. Hospitalizations for gastrointestinal bleeding in 1998 and 2006. Statistical Brief #65. Healthcare Cost and Utilization Project (HCUP) statistical briefs. December 2008.
3. Whelan CT, Chen C, Kaboli P, et al. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147.
4. Wilcox CM, Cryer BL, Henk HJ, et al. Mortality associated with gastrointestinal bleeding events: comparing short-term clinical outcomes of patients hospitalized for upper GI bleeding and acute myocardial infarction in a US managed care setting. Clin Exp Gastroenterol. 2009;2:21-30.
5. Garcia Rodriguez LA, Ruigómez A, Hasselgren G, et al. Comparison of mortality from peptic ulcer bleed between patients with or without peptic ulcer antecedents. Epidemiology. 1998;9(4):452-456.
6. Wilcox CM, Clark WS. Causes and outcome of upper and lower gastrointestinal bleeding: the Grady Hospital experience. South Med J. 1999;92:44-50.
7. Yavorski RT, Wong RK, Maydonovitch C, et al. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol. 1995;90:568–573.
8. Johanson JF. Curbing the costs of GI bleeding. Am J Gastroenterol. 1998;93:1384-1385.
9. Scottish Intercollegiate Guidelines Network (SIGN). Management of acute upper and lower gastrointestinal bleeding. September 2008. www.sign.ac.uk/assets/sign105.pdf.
10. Kim, SK, Cho CD, Wojtowycz AR. The ligament of Treitz (the suspensory ligament of the duodenum): anatomic and radiographic correlation. Abdom Imaging. 2008;33:395-397.
11. Matthew B, et al. Diagnosis of gastrointestinal bleeding: a practical guide for clinicians. World J Gastrointest Pathophysiol. 2014;5(4):467-478.
12. Raju GS, et al. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133(5):1694-1696.
13. Tielleman T, Bujanda D, Cryer B, et al. Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2015;25(3):415-428.
14. Rodriguez-Hernandez H, Rodríguez-Morán M, González JL, et al. Risk factors associated with upper gastrointestinal bleeding and with mortality. Rev Med Inst Mex Seguro Soc. 2009;47(2):179-184.
15. Strate L. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
16. Cocchi MN, Kimlin E, Walsh M, et al. Identification and resuscitation of the trauma patient in shock. Emerg Med Clin N Am. 2007;25:623-642.
17. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-360.
18. Siau K, Chapman W, Sharma N, et alManagement of acute upper gastrointestinal bleeding: an update for the general physician. J R Coll Physicians Edinb. 2017;47:218-230.
19. Rockall T, Logan R, Devlin H, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321.
20. Blatchford O, Murray W, Blatchford M. A risk score to predict need for treatment for uppergastrointestinal haemorrhage. Lancet. 2000;356:1318-1321.
21. Recio-Ramirez, JM, Sánchez-Sánchez Mdel P, Peña-Ojeda JA, et al. The predictive capacity of the Glasgow-Blatchford score for the risk stratification of upper gastrointestinal bleeding in an emergency department. Revista Española de Enfermedades Digestivas. 2015;107(5):262-267.
22. Strate L, Grainek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. Advance online publication March 1, 2016. 2016;111:459-474.
23. Bessa X, O’Callaghan E, Balleste B, et al. Applicability of the Rockall score in patients undergoing endoscopic therapy for upper gastrointestinal bleeding. Dig Liver Dis. 2006;38:12-17.
24. Chaimoff C, Creter D, Djaldetti M. The effect of pH on platelet and coagulation factor activities. Am J Surg. 1978;136(2):257-259.
25. Sreedharan A, Martin J, Leontiadis GI, et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010(7):CD005415.
26. Leontiadis GI , Sharma VK , Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:286-296.
27. Sachar H, Vaidya K, Laine L, et al. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(11):1755-1762.
28. Cheng H, Sheu B. Intravenous proton pump inhibitors for peptic ulcer bleeding: clinical benefits and limits. World J Gastrointest Endosc. 2011;3(3):49-56.
29. Zhang YS, Li Q, He BS, et al. Proton pump inhibitors therapy vs H2 receptor antagonists therapy for upper gastrointestinal bleeding after endoscopy: a meta-analysis. World J Gastroenterol. 2015;21(20):6341-6351.
30. Rahman R, Nguyen D, Sohail U, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta-analysis and systematic review. Ann Gastroenterol. 2016,29:312-317.
31. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:1-46.
32. Issa D, Solomon S, Di Pace B, et al. A Comparison of Azithromycin to Erythromycin Infusions in Improving Visualization of Endoscopy for Upper Gastrointestinal Bleeding. World Congress at ACG2017. Session 2A.
33. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808-1825.
34. Luée A, Lanas A. Protons pump inhibitor treatment and lower gastrointestinal bleeding: balancing risks and benefits. World J Gastroenterol. 2016;22(48):10477-10481.
To comment on this article, contact rdavidson@uspharmcist.com.





