US Pharm. 2018;43(8):34-36.
Hepatitis B is a serious infection of the liver that may be transmitted via bodily fluids including semen, vaginal secretions, and blood. High-risk patients, such as blood and tissue donors, infants born to hepatitis B virus (HBV)–infected mothers, men who have sex with men, and inmates at correctional facilities, should be tested whether they are previously vaccinated or asymptomatic.1 There are approximately 248 million HBV carriers in the world, and 600,000 individuals die annually from HBV-related disease.2 Additionally, hepatitis B may exist as an acute or chronic condition. The acute phase includes icteric hepatitis, fulminant hepatitis, and subclinical hepatitis, and the chronic phase encompasses hepatocellular carcinoma and cirrhosis.
Epidemiology
It is estimated that 2 billion people currently have or have been infected with HBV. Additionally, 248 million individuals are positive for the hepatitis B surface antigen; antigen prevalence is based on geographical area.3
Diagnosis
Aspartate transaminase (AST) and alanine transaminase (ALT) are generally elevated in HBV infection. Since hepatitis B is a viral infection, ALT will increase considerably more than AST. If clinical signs are noted or suspicion of infection is high, several diagnostic tests can confirm viral presence: hepatitis B envelope antigen (HBeAg), antibodies against the envelope (anti-HBe), HBV viral load, and ALT. If HBeAg is present, the virus is still active in the liver and can be transmitted to other individuals; however, the virus is considered to be inactive if HBeAg is negative and anti-HBe is positive.4 HBV viral load measures the amount of virus in a blood sample and is important when assessing viral replication or response to treatment. Although these methods have been effective, more recent assays improve the probability of diagnosis and quantification.5
Device
Aptima: Designed by Hologic, the Aptima HBV Quant Assay is an in vitro nucleic acid amplification test (FIGURE 1). Amplification is defined as massive replication of genetic materials, especially of a gene or DNA sequence. Aptima is indicated to monitor patients taking antiviral therapy for HBV infection. However, the device is not approved as a diagnostic test to confirm the presence of HBV infection or as a screening tool for HBV DNA in blood or blood products.6
Panther: Used in conjunction with Aptima, the Panther System analyzes and determines the concentration of HBV DNA by comparing the results to a calibration curve. It consolidates a broad-assay menu on a single, fully automated platform that enables testing for infectious pathogens.7
Efficacy
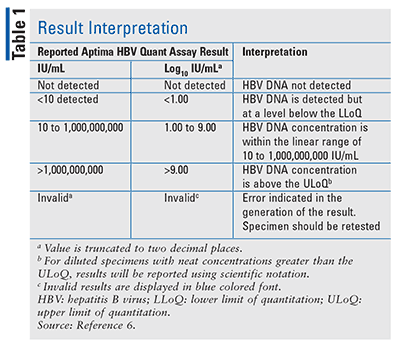
A study was designed to assess the Aptima HBV Quant assay’s ability to predict biochemical, serologic, and virologic clinical-utility endpoints at 48 weeks after initiation of therapy. Specimens were collected prospectively for participants who were prescribed monotherapy with tenofovir or entecavir for chronic HBV infection. There were 331 individuals enrolled in the study; 86 were not evaluated due to withdrawal, discontinuation, low baseline viral loads, or missing Week 48 results. The 245 remaining subjects were monitored for at least one of the clinical-utility endpoints. Concentrations of HBV DNA were reported in IU/mL and log10 IU/mL; interpretation of results may be found in TABLE 1.8 Findings demonstrated that Aptima can quantify HBV DNA levels at baseline during treatment to aid in assessing viral response. The study also confirmed that the predictors of final virologic response varied by treatment and week.9,10 As such, Aptima can be effectively used in the management of HBV-infected patients.
Conclusion
The Aptima HBV Quant assay was developed to help effectively monitor patients with chronic HBV infections who have been prescribed antiviral therapy. However, it should not be used to confirm the presence of HBV infection or screen for the presence of HBV DNA in blood or blood products. More information on this device may be found at www.hologic.com.
REFERENCES
1. Beckett GA, Ramirez G, Vanderhoff A, et al; CDC. Early identification and linkage to care of persons with chronic hepatitis B virus infection—three U.S. sites, 2012-2014. MMWR Morb Motal Wkl Rep. 2014;63(18):399-401.
2. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582-592.
3. Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA.1990;263:1218.
4. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. www.aasld.org/sites/default/files/guideline_documents/hep28156.pdf. Accessed June 2018.
5. Aspinall EJ, Hawkins G, Fraser A, et al. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond). 2011;61(8):531-540.
6. Aptima HBV Quant Assay [package insert]. San Diego, CA: Hologic, Inc.; 2018.
7. Collection, Transport, Preparation, and Storage of Specimens for Molecular Methods; Approved Guideline. CLSI Document MM13-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2005.
8. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. CLSI document EP06-A. Clinical and Laboratory Standards Institute. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2003.
9. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline. 2nd ed. CLSI Document EP17-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
10. Ratnam S, Jang D, Gilchrist J, et al. Workflow and maintenance characteristics of five automated laboratory instruments for the diagnosis of sexually transmitted infections. J Clin Microbiol. 2014;52(7):2299-2304.
To comment on this article, contact rdavidson@uspharmacist.com.





