US Pharm. 2017;42(12):HS2-HS6.
ABSTRACT: Diarrhea is often the revealing symptom in patients suffering from carcinoid syndrome secondary to a carcinoid tumor. The etiology of this burdensome diarrhea is thought to be hypersecretion of serotonin from neuroendocrine cells. Diagnosis is made based on clinical symptoms and two biomarkers, 5-hydroxyindoleacetic acid (found in the urine) and chromogranin A (found in the serum). Synthetic somatostatin analogues (SSAs) have been a staple of therapy for years and continue to be used. Telotristat is the first oral medication indicated in combination with SSAs for patients with carcinoid syndrome diarrhea who no longer respond to SSAs alone.
Neuroendocrine tumors (NETs) encompass a wide variety of slow-growing tumors. The incidence in the United States ranges from two to five per 100,000 patients and appears to be rising, probably because of better screening techniques.1 Although these tumors are generally asymptomatic, symptoms are more likely to develop when a tumor metastasizes to the liver or lung or if the primary tumor originates midgut—i.e., small intestine, appendix, or proximal large bowel.2,3
Carcinoid Tumors and Carcinoid Syndrome
Carcinoid tumors are NETs that primarily arise from enterochromaffin cells located throughout the gastrointestinal (GI) and bronchial tracts.1,2 These cells are responsible for the production, storage, and secretion of many biologically active substances—including serotonin, norepinephrine, dopamine, histamine, bradykinin, prostaglandins, substance P, and others—that may lead to the development of carcinoid syndrome, a collective term encompassing a variety of symptoms mediated by the hormones secreted by the carcinoid tumor.1-4
A well-differentiated NET expresses numerous somatostatin receptors (SSTRs).1 Of the five types of SSTRs, SSTR1 and SSTR5 are targeted by synthetic somatostatin analogues (SSAs). Carcinoid tumors overexcrete serotonin, which exhausts endogenous tryptophan. A normal cell converts only 1% of tryptophan to serotonin, whereas a carcinoid tumor cell produces serotonin until stores of tryptophan are depleted.3 Tryptophan is a precursor of niacin, which is an essential component of nicotinamide and nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate, as well as protein synthesis.3
The most common symptoms of carcinoid syndrome are flushing, diarrhea, tachycardia, bronchospasm, and shortness of breath. Hemodynamic instability may also occur, although this is rare with current therapies.5 Patients with long-term uncontrolled carcinoid syndrome may develop pellagra, cardiac valvular lesions, venous telangiectasia, and/or bronchospasm.2 Fewer than 10% of patients with GI carcinoid tumors develop carcinoid syndrome, and most of those who do have liver metastases.2
Carcinoid Syndrome Diarrhea
Diarrhea occurs in 80% of patients with carcinoid syndrome.6 Patients with carcinoid syndrome diarrhea experience explosive, watery, loose stools multiple times a day, which can be burdensome for the patient and challenging for the healthcare provider. One cohort study found that healthcare costs are 1.5-fold higher and the risk of hospitalization is twofold higher in patients with carcinoid syndrome diarrhea than in those without diarrhea.7 The pathophysiology of carcinoid syndrome diarrhea involves the presence of excess serotonin, which stimulates secretion of colonic mucus, increases peristalsis, and inhibits absorption in the GI tract—all of which lead to diarrhea.3,4,6 Compared with healthy patients, proximal-colon transit time may be up to six times faster and small-bowel transit time may be twice as fast in patients with carcinoid syndrome diarrhea.6
Risk Factors
NETs are thought to develop sporadically or in genetically predisposed individuals.3 When a first-degree relative has an NET, the chance of developing an NET is 12.5 per 100,000 persons and 15.7 per 100,000 persons for men and women, respectively.7 Other associated conditions, which were reported in a retrospective review of 36 patients with carcinoid syndrome diarrhea, include atrophic gastritis (67%), pernicious anemia (58%), hypothyroidism (39%), diabetes (19%), adrenocortical insufficiency (6%), and hyperparathyroidism (6%).8 According to a prospective investigation of 494,000 subjects enrolled in the NIH-AARP Diet and Health Study who were followed for 8 years, saturated fat intake is associated with an increased risk of developing carcinoid tumors.9 Intakes of meat and fat were estimated from food-frequency questionnaires. The risk of carcinoid tumors of the small intestine was associated with high saturated-fat intake compared with low saturated-fat intake (hazard ratio 3.18; 95% CI, 1.62-6.25).9 Once a patient has carcinoid syndrome diarrhea, diet and stress can impact symptoms. Patients should avoid precipitating factors such as foods containing tyrosine (aged cheese, fermented meat and fish, pickled foods, tofu, chocolate), alcohol, and increases in catecholamines (exercise, exertion).1,2
Diagnosis
Diagnosis of carcinoid syndrome diarrhea is based on symptoms of diarrhea and flushing combined with objective findings of carcinoid tumor through biopsy, multiphase abdominal/pelvic CT, MRI, or biomarkers.10 The biomarkers 5-hydroxyindoleacetic acid (5-HIAA; found in the urine) and chromogranin A (CgA; found in the serum) have acceptable specificity and sensitivity for carcinoid tumors.10,11 Serotonin is metabolized by monoamine oxidase to 5-HIAA, and CgA is released from the walls of vesicles that store serotonin. Normal urine 5-HIAA levels range from 3 to 15 mg/24 h; an elevated level has 100% specificity and 73% sensitivity for carcinoid tumors.11 Serum CgA levels tend to correlate with tumor bulk, but not with symptoms. CgA levels greater than 130 mcg/L are associated with 98.4% specificity and 62.9% sensitivity to carcinoid tumors.11 Patients should avoid serotonin-rich foods 48 hours prior to urine collection. Urine levels of 5-HIAA can be falsely lowered by levodopa, whereas serum CgA levels can be falsely elevated by proton pump inhibitors, renal insufficiency, or inflammatory conditions.10-13
Treatment
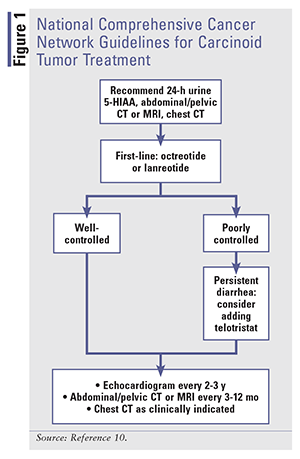
Treatment modalities for carcinoid syndrome diarrhea focus on ameliorating symptoms and modifying the disease itself, as evidenced by normalization of biomarkers.11 SSAs are the mainstay of therapy for carcinoid tumors. Somatostatin influences several GI functions, including GI motility, secretion of pancreatic and intestinal hormones, and secretion of bile and colonic fluids.13 When binding to receptors, somatostatin inhibits secretion of biologic mediators, which dramatically improves flushing and diarrhea by lowering serotonin, as shown by urine 5-HIAA reductions.13 Tumor regression is also seen with SSAs.12,14,15 See FIGURE 1 for the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of carcinoid tumors.
Somatostatin Analogues: The SSAs octreotide and lanreotide provide hormonal control in patients with carcinoid tumors.16,17 Short-acting (SA) octreotide was the first SSA approved for the control of symptoms associated with carcinoid tumors.16,18 Unfortunately, with a half-life of 2 hours, repeated dosing is necessary. Octreotide long-acting (LA) release provides sustained drug levels and is administered IM monthly.15 Studies comparing the SA and LA formulations have demonstrated similar efficacy in terms of symptom control, with response rates of 60% to 72%.18
Lanreotide is available as a prolonged-release formulation (Somatuline Depot) given by deep SC injection every 4 weeks.17,18 SA octreotide and LA lanreotide have similar efficacy in providing symptom relief.16 A phase III clinical trial was conducted to assess the use of SA octreotide SC to control symptoms associated with carcinoid syndrome in patients receiving lanreotide 120 mg SC (n = 59) or placebo (n = 56) every 4 weeks for 16 weeks.19 The percentage of days with octreotide as rescue medication was significantly lower in lanreotide patients (33.7%; 95% CI, 25%-42.4%) than in placebo patients (48.5%; 95% CI, 39.6%-57.4%, P = .016).
Pasireotide (Signifor) binds to SSTR1 and SSTR5 at a higher affinity than octreotide and lanreotide.16 In a phase II clinical trial, it demonstrated efficacy in patients with advanced NETs and carcinoid syndrome who no longer responded to octreotide.20 Pasireotide long-acting release (LAR), which is FDA-approved only for acromegaly and Cushing syndrome, showed similar efficacy in controlling carcinoid syndrome symptoms compared with high-dose octreotide LAR and resulted in improved progression-free-survival in patients refractory to SSAs in a phase III study from 2015.21,22
When symptom control is the goal, therapy begins with SA octreotide. Because it may take up to 3 weeks for LA preparations to reach steady-state levels, symptom control may be managed with SA octreotide.15,18 The dosage is initiated low (100-600 mcg daily in 2-4 doses) and is titrated to the lowest effective dose necessary to control symptoms. LA octreotide starts at 20 mg IM intragluteally every 4 weeks, with SA octreotide given concomitantly for at least 2 weeks. See TABLE 1 for dosing, adverse events, and potential drug-drug interactions.
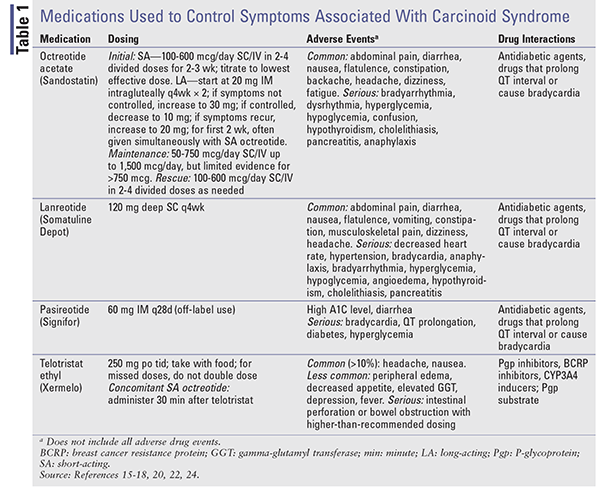
Telotristat Ethyl: Telotristat ethyl (Xermelo), which is a prodrug of telotristat, inhibits the enzyme tryptophan hydroxylase, leading to decreased peripheral serotonin. It is FDA-approved for the treatment of carcinoid syndrome diarrhea in combination with SSA therapy in patients inadequately controlled on SSA therapy alone.23,24 A phase III prospective, randomized study was conducted in which 135 patients experiencing four or more bowel movements daily despite stable-dose SSA received telotristat 250 mg three times daily, telotristat 500 mg three times daily, or placebo for 12 weeks.25 Mean daily bowel-movement frequency reductions for placebo, telotristat 250 mg, and telotristat 500 mg were –0.9, –1.7, and –2.1, respectively. Telotristat is costly, with an average wholesale price of $6,196.80 for eighty-four 250-mg tablets.23
Other Agents: Carcinoid syndrome diarrhea is treated with SSAs, and telotristat is added when symptoms remain uncontrolled. A positive response to chemotherapeutic agents that treat the underlying NET, thereby reducing tumor activity, may alleviate carcinoid syndrome symptoms. It is beyond the scope of this article to review these chemotherapeutic agents. If the disease progresses after the use of octreotide or lanreotide, the NCCN recommends the use of everolimus; interferon alfa-2b; or the cytotoxic chemotherapeutic agents 5-fluorouracil, capecitabine, dacarbazine, oxaliplatin, streptozocin, or temozolomide for unresectable and/or metastatic NETs of the GI tract.10
Conclusion
The incidence of carcinoid tumors, a type of NET, is increasing. There are no known risk factors for the development of NETs or factors that can identify which patients will develop carcinoid syndrome, although most patients with carcinoid syndrome often have liver metastases at onset. Carcinoid syndrome describes a group of symptoms mediated by various hormones released by a carcinoid tumor. Not all patients with carcinoid tumors experience carcinoid syndrome. Carcinoid syndrome diarrhea can be explosive and may occur several times a day. SSAs are the mainstay of therapy for symptomatic relief of diarrhea secondary to carcinoid tumors, and new therapies, such as telotristat added to SSA therapy, provide options for patients whose symptoms are unbearable.
REFERENCES
1. Cives M, Soares HP, Strosberg J. Will clinical heterogeneity of neuroendocrine tumors impact their management in the future? Lessons from recent trials. Curr Opin Oncol. 2016;28:359-366.
2. Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician. 2006;74:429-434.
3. Lips CJ, Lentjes EG, Höppener JW. The spectrum of carcinoid tumours and carcinoid syndromes. Ann Clin Biochem. 2003;40:612-627.
4. Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47-57.
5. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072.
6. von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073-1078.
7. Babovic-Vuksanovic D, Constantinou CL, Rubin J, et al. Familial occurrence of carcinoid tumors and association with other malignant neoplasms. Cancer Epidemiol Biomarkers Prev. 1999;8:715-719.
8. Gough DB, Thompson GB, Crotty TB, et al. Diverse clinical and pathologic features of gastric carcinoid and the relevance of hypergastrinemia. World J Surg. 1994;18:473-479.
9. Cross A, Leitzmann MF, Subar AF, et al. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res. 2008;68:9274-9279.
10. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®): neuroendocrine tumors. Version 3.2017. www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf. Accessed October 11, 2017.
11. Maroun J, Kocha W, Kvols L, et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract. A statement from a Canadian national carcinoid expert group. Curr Oncol. 2006;13:67-76.
12. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
13. van der Lely AJ, de Herder WW. Carcinoid syndrome: diagnosis and medical management. Arq Bras Endocrinol Metabol. 2005;49:850-860.
14. Cives M, Strosberg J. Treatment strategies for metastatic neuroendocrine tumors of the gastrointestinal tract. Curr Treat Options Oncol. 2017;18:14.
15. Enzler T, Fojo T. Long-acting somatostatin analogues in the treatment of unresectable/metastatic neuroendocrine tumors. Semin Oncol. 2017;44:141-156.
16. Octreotide. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2017. http://online.lexi.com. Accessed October 11, 2017.
17. Lanreotide. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2017. http://online.lexi.com. Accessed October 11, 2017.
18. Narayanan S, Kunz PL. Role of somatostatin analogues in the treatment of neuroendocrine tumors. Hematol Oncol Clin N Am. 2016;30:163-177.
19. Vinik AI, Wolin EM, Liyanage N, et al. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22:1068-1080.
20. Kvols LK, Oberg KE, O’Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19:657-666.
21. Wolin EM, Jarzab B, Eriksson B, et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075-5086.
22. Pasireotide. Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics. www.micromedexsolutions.com. Accessed October 23, 2017.
23. Markham A. Telotristat ethyl: first global approval. Drugs. 2017;77:793-798.
24. Telotristat. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2017. http://online.lexi.com. Accessed October 11, 2017.
25. Kulke MH, Hörsch D, Caplin ME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35:14-23.
To comment on this article, contact rdavidson@uspharmacist.com.





