US Pharm. 2018;43(7):22-26.
ABSTRACT: Statins are a class of medications widely used for primary and secondary prevention of cardiovascular events. With six statins available generically, the selection of an appropriate agent may be determined based on drug-specific factors, including dosing considerations, drug interactions, and adverse events. Individualized patient-care plans can be developed based on data from important clinical studies, differences in guidelines, and current management recommendations for two major adverse events associated with statin use.
The cornerstone of dyslipidemia treatment involves the use of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, more commonly known as statins. With a variety of generic options on the market and strong evidence backing their use, statins are some of the most widely prescribed medications in the world.1 However, frequent use of these agents has led to continual scrutiny of their safety and ongoing debate about their role in therapy. This article, which focuses on generic statins, will highlight the debate about statins’ role in therapy, discuss proper use, and explore evidence surrounding two major adverse events.
AVAILABILITY
Of the seven different statins on the market, six are available generically (TABLE 1).2-7 Three available branded formulations are pitavastatin (Livalo), simvastatin oral suspension (FloLipid), and lovastatin extended-release (Altoprev).8-10 The role of branded agents in therapy is limited given the widespread availability of generic options.
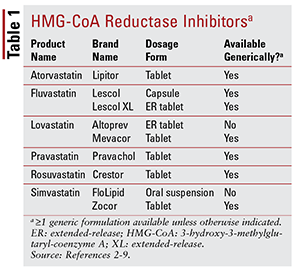
Atorvastatin and simvastatin are also available generically in several combination products. These include the combination of atorvastatin and amlodipine, a calcium channel blocker (CCB) indicated for hypertension, and the combination of simvastatin and ezetimibe, an intestinal cholesterol-absorption inhibitor also indicated for hypercholesterolemia.11,12 Coformulated statin and niacin products (niacin/lovastatin and niacin/simvastatin) were removed from the market in 2016 based on a lack of evidence that adding niacin in statin-treated patients further reduces cardiovascular (CV) outcomes beyond what is seen with statins alone.13
TREATMENT RECOMMENDATIONS
Statins have repeatedly been proven effective for reducing LDL-cholesterol (LDL-C) and triglyceride levels while raising HDL-cholesterol (HDL-C) levels.14 Additionally, they have been shown to improve meaningful patient-oriented outcomes, such as major CV events (CVEs), in both primary and secondary prevention.15-19
Several heavily debated treatment guidelines discuss the role of statins in treating hyperlipidemia. These include the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline and the 2017 American Academy of Clinical Endocrinology (AACE) guideline.14,20 Both guidelines suggest that after implementation of lifestyle modifications, including smoking cessation, adhering to an exercise regimen, and following a heart-healthy diet, statins are first-line therapy for both primary and secondary prevention of CV disease (CVD).14,20 The guidelines differ, however, in how to determine which statins are appropriate for which patients.
The 2013 ACC/AHA guideline introduced four treatment groups to be targeted for statin therapy:
1. Patients with prior atherosclerotic CVD (ASCVD), including those with a prior event (i.e., acute coronary syndromes, history of myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease of presumed atherosclerotic origin).
2. Patients with no history of an ASCVD event who have an LDL-C level of 190 mg/dL or higher. Age is not a basis for exclusion from this criterion.
3. Patients aged 40 to 75 years with diabetes but no history of an ASCVD event, and an LDL-C level between 70 and 189 mg/dL.
4. Patients aged 40 to 75 years without diabetes who have no history of an ASCVD event and an LDL-C level between 70 and 189 mg/dL.20
The ACC/AHA guideline also introduced the idea of grouping statins by intensity (i.e., their expected LDL-C–lowering ability) (TABLE 2).20 Based on the treatment group (as defined above), the guideline recommends specific statin intensities for particular clinical situations (TABLE 3).20 Several treatment groups require calculation of an ASCVD risk score using an ASCVD risk estimator. This online tool takes into account the patient’s age, sex, race, systolic blood pressure, total cholesterol, HDL-C, and past medical history significant for hypertension treatment, diabetes, or smoking.21 Once all inputs have been entered, the patient’s 10-year risk of experiencing an ASCVD event is calculated.
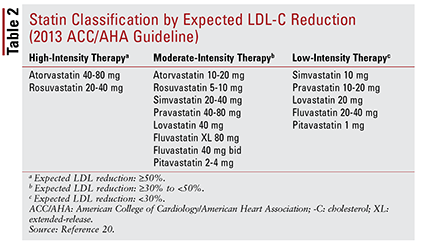
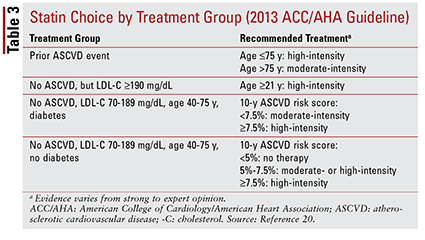
These recommendations do not automatically apply to certain subgroups of patients. For instance, the use of statins in older adults should be approached cautiously. Although statin use for secondary prevention in elderly patients confers significant benefit, its use for primary prevention is unclear.22-24 Additionally, patients with heart failure have not been found to experience the same benefits from statin therapy as those without heart failure.25 Based on these findings, treatment recommendations include limiting initiation of high-intensity statins to patients aged younger than 75 years and possibly avoiding statins in patients with heart failure.20 Overall, the decision to use statins in these groups is not clear-cut and should involve an ongoing conversation between provider and patient about potential risks and benefits of therapy.20
Contrary to the 2013 ACC/AHA recommendation to initiate therapy according to treatment-group classification, the 2017 AACE guideline recommends that individual patients be treated based on laboratory findings.14 According to this guideline, patient risk is determined by a variety of major, additional, and nontraditional risk factors. Individuals are then classified into one of five risk groups, each with a corresponding LDL-C and non–HDL-C goal (TABLE 4). Based on current levels and individualized goals, statins alone or in combination with other agents should be initiated based on expected LDL-C– or non–HDL-C–lowering ability.14
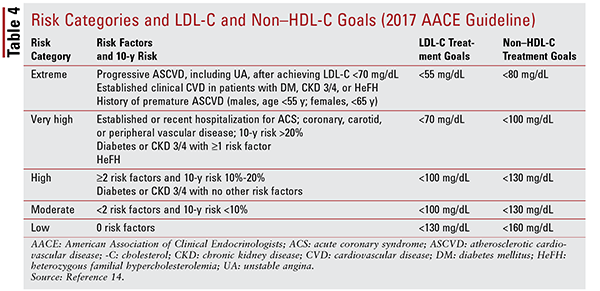
Combination treatment involving the addition of a second agent to ongoing statin therapy remains a somewhat controversial topic. Whereas earlier trials and guidelines minimized the addition of other agents to background statin therapy, recent studies and consensus statements—including one from the ACC/AHA—support the use of ezetimibe, evolocumab, or alirocumab, the last two of which are proprotein convertase subtilisin/kexin type 9 inhibitors, as add-on therapy for secondary prevention.20,26-31 That said, statins remain universal first-line therapy in both primary and secondary prevention; the definitive role of other specific agents remains unclear.
STATIN SELECTION
The choice of statin therapy begins with the clinician’s preferred treatment approach, whether that is the ACC/AHA method of statin treatment groups or the AACE method of LDL-C or non–HDL-C goals. From there, properties of each statin must be taken into account to determine the best option for the individual patient. Not all statins are equal, and several key differences exist that may influence patient selection, including dosing considerations, drug interactions, and adverse events (AEs).
Dosing
Although most statins may be taken without regard to meals, immediate-release (IR) lovastatin should be taken with the evening meal because of increased bioavailability.4 However, the opposite is true for extended-release lovastatin, which should not be taken with food owing to decreased bioavailability.8 Further, although all statins may be dosed once daily, IR fluvastatin and lovastatin may require twice-daily dosing.3,4 Finally, whereas atorvastatin, pravastatin, and rosuvastatin may be administered at any time of day, fluvastatin, lovastatin, and simvastatin should be taken in the evening.3,4,7 Simvastatin, in particular, was shown to have significantly different efficacy when taken in the evening as opposed to the morning.32
Drug Interactions
Each statin has different concerns regarding drug-drug and drug-food interactions because of the specific pathways through which each is metabolized (TABLE 5). Too many drug interactions exist to discuss each in detail; therefore, only a selected few are described below.
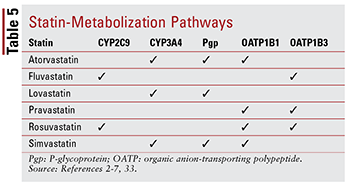
Fibrates: Although the combination of a fibrate and a statin has not been shown to meaningfully alter clinical outcomes, patients may still be prescribed gemfibrozil, fenofibrate, or fenofibric acid together with a statin to target severely uncontrolled hypertriglyceridemia.14,20,26,33 Because both of these classes are associated with muscle-related toxicity, combination therapy significantly increases this risk compared with either therapy alone.33 This additive toxicity is more frequently seen with gemfibrozil than with other fibrates.33 Therefore, gemfibrozil use is contraindicated with simvastatin and should be avoided with other statins.7,20 If a statin and a fibrate will be used together, fenofibrate or fenofibric acid is preferred.20,33 However, if gemfibrozil must be used, fluvastatin—or, with careful monitoring, atorvastatin or rosuvastatin (maximum 10 mg daily)—is the agent of choice.33
Amiodarone: This antiarrhythmic agent indicated for ventricular fibrillation is a known inhibitor of P-glycoprotein (Pgp) and the CYP450 enzyme system, specifically CYP3A4 and, to a lesser extent, CYP2C9.33,34 As a result, maximum recommended dosages exist for lovastatin (maximum 40 mg daily) and simvastatin (maximum 20 mg daily) when used with amiodarone.4,7,33 Although atorvastatin is metabolized through CYP3A4, no dosage adjustment is necessary (similarly to other statins) because data do not suggest serious AEs when atorvastatin and amiodarone are used concomitantly.2,33
CCBs: Both dihydropyridine (amlodipine) and nondihydropyridine CCBs (diltiazem, verapamil) have been shown to have meaningful drug interactions with statins. Similarly to amiodarone, amlodipine, diltiazem, and verapamil inhibit CYP3A4.33,35-37 Amlodipine also has an inhibitory effect on Pgp.33,35 Based on AEs reported in the literature, a maximum daily dosage of 20 mg for lovastatin is recommended when used with amlodipine, diltiazem, or verapamil.4,33 It is also recommended that simvastatin not exceed 20 mg when used with amlodipine, but a lower dose of 10 mg should be administered when used with diltiazem and verapamil.7,33 No specific dosing recommendations exist with atorvastatin, although caution should be exercised when it is administered with either of the nondihydropyridine CCBs.2,33
Adverse Drug Reactions
Statins are generally well tolerated; however, discontinuation rates remain high.38 Discontinuation of statin therapy and failure to resume statin therapy after the occurrence of adverse drug reactions are associated with increased rates of CVEs.39 Patients’ concerns about statin safety in light of two major statin-related AEs—myotoxicity and new-onset diabetes—may lead to statin discontinuation.
Myotoxicity: Statins have long been associated with muscle-related toxicity, including myalgia (muscle pain without elevated creatine kinase [CK]), myopathy (general term for muscle disease), and myositis (muscle inflammation), the last two of which involve significant CK elevations.40,41 All statins share a warning for the rare but serious side effect of rhabdomyolysis.2-7 Often, however, myotoxicity attributed to statins is due to the nocebo effect; that is, a person believes that a medication will cause harm and subsequently the medication causes the anticipated harm.42,43 Therefore, to ensure the best outcome, pharmacists must be well versed in this commonly reported side effect and its proper management.
It is incorrect to assume that all reports of muscle symptoms are due to the nocebo effect; proper vetting of the patient’s past medical history (PMH), description of the pain, and association with statin therapy is imperative.44 First, an examination of PMH for CK elevations and other potential causes of muscle pain or weakness allows the clinician to treat alternative underlying pathophysiology or correct factors that may alter statin metabolism. Common causes include old age, drug interactions, impaired renal or hepatic function, increased physical activity, and vitamin D deficiency.20,41
Next, the clinician should compare the patient’s presentation with commonly seen statin-induced symptoms. Statin-induced muscle toxicity usually presents as pain, tenderness, cramps, and weakness, usually in the leg muscles.20,41 These symptoms are typically worse after exercise and do not resolve without discontinuation of the offending agent.41
The last step is to determine whether a causal relationship between symptoms and statin use exists. When a statin is suspected as the cause of mild-to-moderate symptoms, temporary withdrawal is recommended. If the symptoms do not resolve after approximately 2 weeks, the statin is likely not the cause and should be reinitiated at the original dose. However, if the symptoms resolve, a retrial of the same statin at the same or a lower dose should be undertaken. If similar symptoms are then experienced, the statin should be presumed the cause and discontinued. Upon symptom resolution, a low dose of an alternative statin should be initiated and titrated to the maximum tolerated dose.20,41 Patient acceptance and understanding of this process are important because the nocebo effect may otherwise lead to further perceived reactions.
Several other strategies exist for managing and preventing these symptoms. First, owing to a higher likelihood of symptoms, the clinician may choose to avoid the most lipophilic statins (lovastatin and simvastatin) in favor of more hydrophilic statins (fluvastatin, pravastatin, and rosuvastatin).44 Simvastatin 80 mg should never be initiated as new therapy in any patient because of an unusually high frequency of statin-associated muscle symptoms.7,45 Alternative therapies, such as coenzyme Q10, have not demonstrated consistent benefit but may be considered in patients experiencing psychologically induced symptoms.41,44,46 Finally, extended-interval dosing, which involves dosing statin medications several times weekly rather than once daily, may be considered. This approach improves adherence and lipid profiles in patients with prior statin intolerance.47-49
New-Onset Diabetes: An important AE associated with statin use is new-onset diabetes. Statins have been shown to increase the risk of diabetes development, although several important caveats exist.50 First, although this can be considered something of a class effect, the true risk of each specific statin is still unknown.51,52 In the largest meta-analysis to date, pravastatin had the lowest risk, simvastatin and atorvastatin had a moderate risk, and rosuvastatin had the highest risk of causing new-onset diabetes.53 None of these findings was statistically significant, however.53 Additionally, the finding that diabetes risk increases as the dose of statin increases has been inconsistent.54
Statin-induced diabetes is most prevalent in persons already at high risk for developing diabetes.50,54 This includes older patients and those with prediabetes or metabolic syndrome.50,55 One consistent finding has been that, for each new case of incident diabetes caused by statins, several CVEs can be prevented in higher-risk patients.50 Therefore, pharmacists should counsel anxious statin users at moderate or high risk for CV complications that the risk of developing diabetes is more than offset by the CV risk-reduction benefits. However, the risk-benefit ratio is unclear in patients with a very low risk of CVEs.50
CONCLUSION
Although statin therapy is not without risks, its benefits in reducing CV outcomes have made it the cornerstone of CVE prevention. Available guidelines differ widely, but the one constant is that statins should be first-line therapy for primary and secondary prevention in nearly every patient. The widespread generic availability of statins has made these agents increasingly more accessible. By understanding that not all statins are the same, the pharmacist can help ensure the best possible outcome for each patient.
REFERENCES
1. IMS Institute for Healthcare Informatics. Medicines use and spending in the U.S.: a review of 2015 and outlook to 2020. https://morningconsult.com/wp-content/uploads/2016/04/IMS-Institute-US-Drug-Spending-2015.pdf. Accessed March 18, 2018.
2. Lipitor (atorvastatin) package insert. New York, NY: Pfizer, Inc; June 2017.
3. Lescol/Lescol XL (fluvastatin) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; August 2017.
4. Mevacor (lovastatin) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2014.
5. Pravachol (pravastatin) package insert. Princeton, NJ: Bristol-Myers Squibb Company; July 2016.
6. Crestor (rosuvastatin) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; August 2017.
7. Zocor (simvastatin) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2015.
8. Altoprev (lovastatin) package insert. Zug, Switzerland: Covis Pharma; April 2017.
9. FloLipid (simvastatin) package insert. Brooksville, FL: Salerno Pharmaceuticals LP; July 2017.
10. Livalo (pitavastatin) package insert. Montgomery, AL: Kowa Pharmaceuticals America, Inc; November 2016.
11. Caduet (amlodipine/atorvastatin) package insert. New York, NY: Pfizer Inc; October 2017.
12. Vytorin (ezetimibe/simvastatin) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2018.
13. AbbVie Inc.; withdrawal of approval of new drug applications for Advicor and Simcor. www.federalregister.gov/documents/2016/04/18/2016-08894/abbvie-inc-withdrawal-of-approval-of-new-drug-applications-for-advicor-and-simcor. Accessed March 18, 2018.
14. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87.
15. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435.
16. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207.
17. Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581-590.
18. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192.
19. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
20. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
21. American College of Cardiology. ASCVD Risk Estimator Plus. http://tools.acc.org/ASCVD-Risk-Estimator. Accessed March 18, 2018.
22. Wenger NK, Lewis SJ, Herrington DM, et al. Outcomes of using high- or low-dose atorvastatin in patients 65 years of age or older with stable coronary heart disease. Ann Intern Med. 2007;147:1-9.
23. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
24. Teng M, Lin L, Zhao YJ, et al. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta-analysis. Drugs Aging. 2015;32:649-661.
25. Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
26. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
27. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431-1443.
28. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
29. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
30. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC Expert Consensus Decision Pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785-1822.
31. ODYSSEY Outcomes: results suggest use of PCSK9 inhibitor reduces CV events, LDL-C in ACS patients. American College of Cardiology. www.acc.org/latest-in-cardiology/articles/2018/03/05/15/53/sat-9am-odyssey-outcomes-cv-outcomes-with-alirocumab-after-acs-acc-2018. Accessed June 20, 2018.
32. Wallace A, Chinn D, Rubin G. Taking simvastatin in the morning compared with in the evening: randomised controlled trial. BMJ. 2003;327:788.
33. Wiggins BS, Saseen JJ, Page RL, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134:e468-e495.
34. Pacerone (amiodarone) package insert. Maple Grove, MN: Upsher-Smith Laboratories LLC; July 2017.
35. Norvasc (amlodipine) package insert. New York, NY: Pfizer Inc; October 2017.
36. Cardizem (diltiazem) package insert. Bridgewater, NJ: Valeant Pharmaceuticals; November 2016.
37. Calan (verapamil) package insert. New York, NY: Pfizer Inc; September 2017.
38. Riaz H, Khan AR, Khan MS, et al. Meta-analysis of placebo-controlled randomized controlled trials on the prevalence of statin intolerance. Am J Cardiol. 2017;120:774-781.
39. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med. 2017;167:221-227.
40. Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63:859-866.
41. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012-1022.
42. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
43. Pedro-Botet J, Rubiés-Prat J. Statin-associated muscle symptoms: beware of the nocebo effect. Lancet. 2017;389:2445-2446.
44. Laufs U, Filipiak KJ, Gouni-Berthold I, et al. Practical aspects in the management of statin-associated muscle symptoms (SAMS). Atheroscler Suppl. 2017;26:45-55.
45. FDA Drug Safety Communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed March 18, 2018.
46. Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24-34.
47. Goldberg AS, DeGorter MK, Ban MR, et al. Efficacy and plasma drug concentrations with nondaily dosing of rosuvastatin. Can J Cardiol. 2013;29:915-919.
48. Matalka MS, Ravnan MC, Deedwania PC. Is alternate daily dose of atorvastatin effective in treating patients with hyperlipidemia? The Alternate Day Versus Daily Dosing of Atorvastatin Study (ADDAS). Am Heart J. 2002;144:674-677.
49. Copher HR, Stewart RD. Daily dosing versus alternate-day dosing of simvastatin in patients with hypercholesterolemia. Pharmacotherapy. 2002;22:1110-1116.
50. Rochlani Y, Kattoor AJ, Pothineni NV, et al. Balancing primary prevention and statin-induced diabetes mellitus prevention. Am J Cardiol. 2017;120:1122-1128.
51. Corrao G, Ibrahim B, Nicotra F, et al. Statins and the risk of diabetes: evidence from a large population-based cohort study. Diabetes Care. 2014;37:2225-2232.
52. Lim S, Oh PC, Sakuma I, Koh KK. How to balance cardiorenometabolic benefits and risks of statins. Atherosclerosis. 2014;235:644-648.
53. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
54. Crandall JP, Mather K, Rajpathak SN, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care. 2017;5:e000438.
55. Ganda OP. Statin-induced diabetes: incidence, mechanisms, and implications. F1000Res. 2016;5.
To comment on this article, contact rdavidson@uspharmacist.com.






