US Pharm. 2017;42(2):32-35.
ABSTRACT: Atrial fibrillation (AF) is the most common cardiac arrhythmia in the United States. It is a complicated disease state involving an irregular heart rate and rhythm. AF generally affects the aging population and is classified as either valvular AF (VAF) or nonvalvular AF (NVAF). The two differ based on the patient’s valve status and treatment strategies. AF treatment is directed toward rate control, with or without rhythm control, and anticoagulation for prevention of cardioembolic stroke. Several direct oral anticoagulants (edoxaban, apixaban, rivaroxaban, dabigatran) have recently gained approval for use in NVAF. Pharmacists should educate patients about potential drug-drug interactions and adverse effects with this class of medications.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting about 5.2 million Americans in 2010, and that number is projected to increase to 12.1 million by 2030.1 AF is seen primarily in elderly patients, with <1% of patients under the age of 60 years. AF increases the risk of stroke, heart failure, dementia, and death.2 Signs of AF include irregular heart rate and atrial activity on an ECG;2 patients may also experience symptoms of ventricular tachycardia, shortness of breath, and fatigue. Symptoms may be more severe when cardiac comorbidities, such as heart failure, are present.3
In AF, structural or electrical abnormalities affect atrial tissues, resulting in the generation of irregular electrical impulses and subsequent ineffective atrial contraction.2 Although AF is classified as valvular (VAF) or nonvalvular (NVAF), the distinction between the two forms is still debated in the literature.4 The difference may be related more to the pathogenesis of thromboembolism than to the presence or absence of valve irregularities.4
Patients with VAF often have rheumatic mitral stenosis, mechanical or bioprosthetic heart valves, or mitral valve repair. The recommended anticoagulant for prevention of thromboembolic events in these patients is warfarin, a vitamin K antagonist (VKA). Although the direct oral anticoagulants (DOACs) are not approved for use in VAF, many of the studies of these agents did include patients with native valve disease other than mitral stenosis, and only patients with moderate-to-severe mitral stenosis or prosthetic valves were excluded. NVAF patients have rhythm irregularities unrelated to any artificial valve or valvular disorder, and may be treated with aspirin, warfarin, or a DOAC depending on the risk of stroke or systemic embolism.2,4
Anticoagulant Treatment
Pharmacologic management of AF is directed primarily at ventricular rate control. Based on patient-specific risk factors and symptoms, rhythm control and anticoagulation for prevention of cardioembolic stroke may also be indicated. Patients with AF may be at risk for thromboembolism due to incomplete pumping of blood from the atria, which leads to pooling of blood and an increased risk of clot formation. If dislodged, clots can cause significant systemic embolic events, primarily resulting in cardioembolic stroke.3
According to the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) Guidelines for the Management of Patients with Atrial Fibrillation, there are many anticoagulation options to prevent systemic embolic events.2 A CHA2DS2-VASc score (TABLE 1), which calculates the risk of stroke in patients with AF, is used to help determine the overall risk for the patient to develop a thromboembolic event and which anticoagulant therapy would be most appropriate for prevention.2
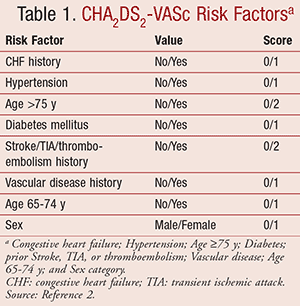
For NVAF, warfarin or a DOAC is indicated to prevent thrombotic events in patients with a history of stroke, transient ischemic attack (TIA), or a CHA2DS2-VASc score ≥2. If the goal international normalized ratio (INR) of 2.0 to 3.0 cannot be maintained in patients receiving warfarin, a DOAC such as edoxaban, apixaban, rivaroxaban, or dabigatran may be used.2
DOACs are newer oral anticoagulants that exhibit different mechanisms of action from warfarin. Edoxaban, apixaban, and rivaroxaban inhibit factor Xa in the coagulation cascade, thus reducing thrombin generation and subsequent thrombus formation.5 The fourth DOAC, dabigatran, is a direct inhibitor of thrombin. Thrombin is required for the conversion of fibrinogen into fibrin. Inhibition of this step of the coagulation cascade prevents thrombus formation.5 Advantages of DOACs over anticoagulation with warfarin include fixed dosing and no need for INR monitoring, although renal function should be checked periodically. In comparison, VKAs such as warfarin require patient-specific dosing and frequent monitoring of INR.4 Dosing for the DOACs is listed in TABLE 2.

There are currently no approved agents for reversal of anticoagulant effects of the factor Xa inhibitors. Kcentra (prothrombin complex concentrate) was approved in 2013 for reversal of anticoagulation in patients receiving a VKA.6 While not approved for reversal of factor Xa inhibitors, it has been shown in animals and healthy humans receiving apixaban or rivaroxaban to decrease the time to thrombin formation, and has been administered to patients with factor Xa inhibitor–associated refractory bleeding.7 Idarucizumab (Praxbind) was approved in 2015 for reversal of dabigatran’s anticoagulation effect in cases of emergency surgery and life-threatening, or uncontrolled, bleeding.8
Edoxaban (Savaysa): Edoxaban was approved in 2015 to reduce the risk of stroke and systemic embolism in patients with NVAF.9 It is also approved to treat deep venous thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial parenteral anti-coagulant therapy. It is taken once daily and is available in 15-, 30-, and 60-mg tablets. For the treatment of NVAF, the recommended dose of edoxaban is 60 mg once daily (TABLE 2) in patients with a creatinine clearance (CrCl) >50 to 95 mL/min. In patients with CrCl 15 to 50 mL/min, the recommended dose is 30 mg once daily. Edoxaban may be taken with or without food. Edoxaban should not be used in patients with a CrCl >95 mL/min or <15 mL/min.9
In the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, patients with CrCl >95 mL/min had an increased rate of ischemic stroke with edoxaban 60 mg compared to those receiving warfarin.10 When transitioning to edoxaban from warfarin, discontinue warfarin and initiate therapy with edoxaban when the patient’s INR is ≤2.5. If transitioning from another DOAC to edoxaban, discontinue the previous anticoagulant and administer edoxaban at the next scheduled dose.9
Edoxaban is minimally metabolized by hydrolysis, conjugation, and oxidation via CYP3A4, and is eliminated primarily unchanged in the urine. Edoxaban does not inhibit the major CYP450 enzymes and does induce CYP1A2 or CYP3A4.9 The half-life of edoxaban is 10 to 14 hours following oral administration, and the onset of action is 1 to 2 hours. Major drug interactions include other anticoagulants and rifampin, and concomitant use of these agents should be avoided. Nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents including clopidogrel, prasugrel, and ticagrelor may increase the risk of bleeding. The most common adverse effects are bleeding and anemia. Edoxaban is not recommended in patients with renal failure or severe renal impairment (defined as CrCl <15 mL/min) or in patients with moderate-to-severe hepatic impairment. Edoxaban is Pregnancy Category C and is not recommended for nursing mothers.9
Apixaban (Eliquis): Apixaban was approved in 2012 to reduce the risk of stroke and systemic embolism in patients with NVAF.11 It is also used to treat or prevent DVT. The recommended dose is 5 mg twice daily with or without food (TABLE 2). The dose is reduced to 2.5 mg twice daily for patients with at least two of the following characteristics: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL. Apixaban has been studied in 8 patients with end-stage renal disease (ESRD).12 Based on the study, the package insert was changed to include a statement that serum concentrations in patients with ESRD are similar to those seen in clinical studies but no information is available on the reduction of stroke or bleeding risks in patients with ESRD.11 Apixaban is Pregnancy Category B, with treatment likely to increase the risk of hemorrhage during pregnancy. Its use is not recommended in nursing mothers or in severe hepatic impairment.11
Rivaroxaban (Xarelto): Rivaroxaban was approved in 2011 for the treatment of NVAF and the treatment and prevention of DVT and PE as well as prophylaxis of DVT in patients undergoing knee or hip replacement surgery.13 For NVAF, the recommended dose is 20 mg daily with the evening meal (TABLE 2). Administration of a 20-mg tablet with food resulted in a 76% increase in maximum concentration. For patients with CrCl 15 to 50 mL/min, the recommended dose is 15 mg once daily with the evening meal. The drug should be avoided in patients with CrCl <15 mL/min. Rivaroxaban is Pregnancy Category C, and its use in nursing mothers or in moderate-to-severe hepatic impairment is not recommended.13
Apixaban and rivaroxaban should be avoided with strong inducers and inhibitors of P-glycoprotein (P-gp) and CYP3A4.11,13 Edoxaban is minimally metabolized by CYP3A4 but no information about its use with CYP3A4 inducers or inhibitors is provided. Edoxaban should be avoided with inducers of P-gp such as rifampin.9
Dabigatran (Pradaxa): Dabigatran was approved in 2010 for the treatment of NVAF.14 It also has indications for the treatment and prevention of DVT and PE as well as prophylaxis of DVT in patients undergoing knee or hip replacement surgery. For NVAF, the recommended dose is 150 mg twice daily, with or without food, for patients with CrCl >30 mL/min (TABLE 2). For patients with CrCl 15 to 30 mL/min, the recommended dose is 75 mg twice daily. Dabigatran is Pregnancy Category C, and its use in nursing mothers is not recommended. The manufacturer has not provided dosing recommendations for use in patients with hepatic failure or CrCl <15 mL/min. Unlike the other DOACs, dabigatran causes gastritis-like adverse events in >15% of patients.14
Guideline Recommendations
The 2014 AHA/ACC/HRS guidelines recommend controlling ventricular rate with verapamil or diltiazem, classified as nondihydropyridine calcium channel blockers (non-DHP CCBs), or with a beta-blocker.2 Digoxin may be used in combination with beta-blockers or non-DHP CCBs to improve ventricular rate control during exercise. Amiodarone may be used to control rate when other measures have failed. Non-DHP CCBs should not be used in patients with decompensated heart failure or left ventricular systolic dysfunction (ejection fraction <40%) because of negative inotropic effects per the guidelines.2
Maintenance of normal sinus rhythm may be indicated in patients who experience symptoms of AF despite adequate ventricular rate control. Flecainide, propafenone, amiodarone, dofetilide, dronedarone, and sotalol may be used for maintenance of normal sinus rhythm in patients without underlying structural heart disease or heart failure. As with all drug therapy, risks versus benefits of each rhythm control medication should be weighed before starting treatment based upon the patient’s comorbidities and pertinent drug interactions. If AF becomes permanent, antiarrhythmic drugs should be discontinued.2
If an AF patient has other comorbid conditions, treatment and drug therapy become more complicated. If a patient has AF and an enlarged heart, anticoagulation is indicated regardless of the patient’s CHA2DS2-VASc score.2 Dofetilide or amiodarone are the only agents recommended for rhythm control in patients with AF and concomitant systolic heart failure (left ventricular ejection fraction <40%). These antiarrhythmic drugs should be used with the other medications commonly used in heart failure, including a beta-blocker, loop diuretic, angiotensin-converting enzyme (ACE) inhibitor, and digoxin.2 Renal function should be evaluated before initiation of a DOAC and then at least annually thereafter. Warfarin is the recommended anticoagulant in patients with ESRD.2
Clinical Studies
The REVISIT-US (Real-world EVIdence on Stroke prevention In patients with aTrial fibrillation in the United States) study was a retrospective trial comparing the safety and efficacy of rivaroxaban and apixaban to warfarin in NVAF patients from 2012 to 2014.15 Edoxaban, while directed at the same factor in the coagulation cascade as rivaroxaban and apixaban, was not included because it was not yet FDA-approved. Compared to patients receiving warfarin, those receiving rivaroxaban or apixaban had a significantly reduced incidence of intracranial hemorrhage. However, when compared to warfarin and prevention of ischemic stroke, neither apixaban nor rivaroxaban demonstrated similar efficacy.15
The efficacy and safety of edoxaban were compared to warfarin in the ENGAGE AF-TIMI 48 trial, a random-ized, double-blind, double-dummy study comparing two different daily doses of edoxaban (30 or 60 mg) to warfarin.10 Edoxaban was shown to be noninferior to warfarin for the prevention of the two primary endpoints, stroke and systemic embolic events. Edoxaban demonstrated significantly lower rates of major bleeding, with annualized rates of bleeding at 3.43% for warfarin, 2.75% with high-dose edoxaban, and 1.61% with low-dose edoxaban. Edoxaban was also shown to significantly reduce death from cardiovascular causes—bleeding, stroke, myocardial infarction, and systemic embolic event. Annualized rates of death from a cardiovascular cause were 3.17% with warfarin, 2.74% with high-dose edoxaban, and 2.71% with low-dose edoxaban.10
Pharmacist Involvement
Pharmacists can positively impact outcomes for individuals with AF by assisting their patients to better manage this complicated disease state.16 The importance of adherence to the prescribed medication regimen must be stressed. Patients should be educated about their medications, including potential drug-drug and drug-disease interactions, when the medication is initially dispensed and at the time of refill. Common adverse effects, as well as those that must be reported to the prescriber such as hematuria, hematochezia, and melena, should be discussed with the patient, and patients should be instructed to consult the prescriber and never stop a medication on their own.
Encourage patients to take their medications exactly as stated on the label at around the same time every day. Monitor refill intervals and, if it seems refills are delayed, revisit the importance of adherence to the prescribed therapy, as well as the significance of each prescribed medication in the management of AF. Consult with the patient to ascertain whether any adverse effects or other factors are barriers to adherence (e.g., cost of medication, dosing schedule). When necessary, contact the prescriber or refer the patient to the prescriber to resolve issues of concern. If available, sign patients up for automated refills to encourage adherence and increase quality of life. Inform patients of the importance of keeping an up-to-date list of all their medications with them always, including prescription and nonprescription drugs and herbal supplements. By printing the patient’s current medication profile, pharmacists facilitate the creation of an accurate list.
In addition, pharmacists should screen for drug-drug interactions, remembering to include nonprescription and herbal products as well as prescription medications. When asked for a nonprescription recommendation, pharmacists should ensure that the product does not interact with any other medications the patient may be taking. Screening for drug-drug interactions is essential, as many medications used in the treatment of AF have narrow therapeutic indexes and the potential to cause serious adverse events.
Conclusion
AF is the most common cardiac conduction abnormality. NVAF occurs in patients with native valves and no underlying valvular disease. Several DOACs have recently been approved for NVAF, and these medications provide a therapeutic option for patients who do not want, or are unable, to undergo monitoring of INR or maintain therapeutic INR. DOACs are a vital component of NVAF management, and pharmacists can positively impact patient outcomes by educating patients about their medications and screening for potential adverse drug reactions and interactions.
REFERENCES
1. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142-1147.
2. 2014 AHA/ACC/HRS Guideline for the Management of Patients with AF. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
3. Sanoski CA, Bauman JL. Chapter 8. The Arrhythmias. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689§ionid=48811457. Accessed October 19, 2016.
4. Fauchier L, Philippart R, Clementy N, et al. How to define valvular AF? Arch Cardiovasc Dis. 2015;108(10):530-539.
5. Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thrombotic events. Ther Clin Risk Manag. 2015;11:967-977.
6. Kcentra (prothrombin complex concentrate [human]) prescribing information. Kankakee, IL: Behring LLC; September 2014. http://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf. Accessed November 29, 2016.
7. Baumann Kreuziger LM, Keenan JC, Morton CT, Dries DJ. Management of the bleeding patient receiving new oral anticoagulants: a role for prothrombin complex concentrates. BioMed Res Int. 2014;2014:583794.
8. Praxbind (idarucizumab) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; October 2015. http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf. Accessed November 18, 2016.
9. Savaysa (edoxaban) prescribing information. Parsippany, NJ: Daiichi Sankyo, Inc; January 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf. Accessed November 18, 2016.
10. Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with AF. N Engl J Med. 2013;369(22):2093-2104.
11. Eliquis (apixaban) prescribing information. Princeton, NJ: Bristol-Myers Squibb Co; December 2012. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf. Accessed November 18, 2016.
12. Wang X, Tirucherai G, Marbury T, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56(5)628-636.
13. Xarelto (rivaroxaban) prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc; December 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf. Accessed November 18, 2016.
14. Pradaxa (dabigatran) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; November 2015. http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed November 18, 2016.
15. Coleman CI, Antz M, Bowrin K, et al. Real-world evidence of stroke prevention in patients with nonvalvular AF in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;32(12):2047-2053.
16. Tran HN, Tafreshi J, Hernandez EA, et al. A multidisciplinary atrial fibrillation clinic. Curr Cardiol Rev. 2013;9:55-62.
To comment on this article, contact rdavidson@uspharmacist.com.





