US Pharm. 2018;43(2):22-26.
ABSTRACT: Heart failure (HF) causes significant morbidity and mortality and imposes a large cost burden on the U.S. healthcare system. It has been estimated that the prevalence of HF will increase 46% by the year 2030. For these reasons, hospital readmissions are now being monitored by several agencies. In 2016, the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America published a focused update on new pharmacologic therapy for HF, which was prompted by recent evidence and availability of the novel agents Entresto (sacubitril/valsartan) and Corlanor (ivabradine). Pharmacists are in a unique position to assist in HF care, as the use of specific medications has proven to decrease morbidity, mortality, and hospital readmissions.
An estimated 6.5 million Americans aged 20 years and older are burdened with heart failure (HF). In 2014, HF was listed as the cause of one in every nine deaths. Approximately 50% of patients die 5 years after being diagnosed with HF. The direct medical costs for HF in 2012 were projected to be about $21 billion.1 It has been estimated that the prevalence of HF will increase 46% by the year 2030.2 This means that one in every 33 Americans—numbering more than 8 million people—will have HF. Cost projections for the healthcare system suggest that, in 2030, $53 billion and $70 billion will be attributed to direct medical costs and indirect costs of HF, respectively.2 Roughly 45% of HF patients who are symptomatic have reduced ejection fraction (EF).1 Because of the high rate of readmission for HF, the Centers for Medicare & Medicaid Services and the Hospital Quality Alliance currently require reporting of 30-day hospital readmissions for HF.3 Medicare data from 2015 show that 16.4% of Medicare patients with HF experienced a potentially preventable readmission; this is a decline of 3.1% from 2010.4
HF occurs when the heart pumps an insufficient amount of blood to meet the body’s metabolic demands. Risk factors for HF include atherosclerotic disease, diabetes, hypertension, metabolic syndrome, and smoking. Patients with HF often present with symptoms of dyspnea, edema, and fatigue.5
Left ventricular (LV) dysfunction is classified as impaired ventricular contraction and ejection (systolic dysfunction) and impaired relaxation and ventricular filling (diastolic dysfunction). This, in turn, causes decreased cardiac output, which leads to global hypoperfusion. Hypoperfusion activates compensatory mechanisms to aid in maintaining circulation. These compensatory mechanisms include neurohormonal activation, increased contractility, vasoconstriction, and ventricular hypertrophy and remodeling. Long-term activation of these mechanisms leads to the progression of HF.6
Neurohormonal activation promotes sodium and water retention, and the overstimulation of the sympathetic nervous system and the release of catecholamines increase heart rate and contractility. Activation of the renin-angiotensin-aldosterone system (RAAS) and the release of vasopressin increase vasoconstriction. The chronic hemodynamic stresses on the heart lead to remodeling. As the ventricle continues to enlarge and the myocardium hypertrophies, wall tension and fibrosis increase, further impairing contractility.6
Natriuretic peptides counteract vasoconstriction and are released upon atrial or ventricular stretch. Natriuretic peptides cause vasodilation, excretion of salt and water, and inhibition of renin, aldosterone, and vasopressin secretion. Additionally, endothelium-derived vasoactive substances, such as nitric oxide and bradykinin, act locally to promote vasodilation.6 Natriuretic peptides and bradykinin are degraded by neprilysin, a neutral endopeptidase.7
HF with preserved EF (HFpEF), also referred to as diastolic dysfunction, is defined as clinical signs of HF with an EF of 50% or greater. HFpEF may be further classified as borderline (EF = 41%-49%) and improved (EF >40%). HF with reduced EF (HFrEF), also referred to as systolic dysfunction, is distinguished by an EF of 40% or less.5 There is a paucity of efficacy data on the treatment of HFpEF. Treatment effectiveness has been demonstrated only in patients with HFrEF.
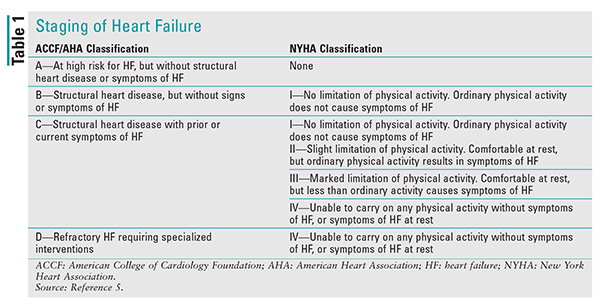
HF staging is broken down into structural and functional classifications. The American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) provide structural classification, and the New York Heart Association (NYHA) provides functional classification. TABLE 1 summarizes staging of HF.5
Updated Guidelines
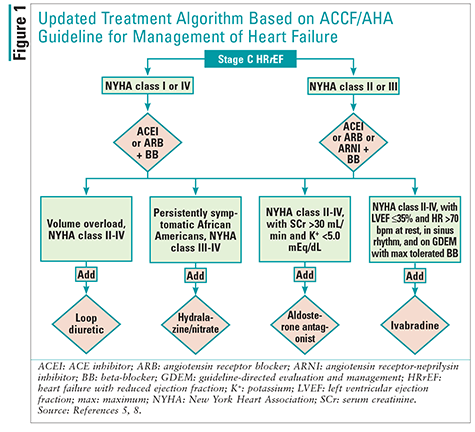
In May 2016, the American College of Cardiology, the AHA, and the Heart Failure Society of America published a focused update on new pharmacologic therapy for HF. The update was spurred by recent evidence regarding HF, and the new drugs sacubitril/valsartan and ivabradine were included in the update. Because these two new agents have evidence only for stage C HFrEF, this was the only section of the guidelines to be updated; recommendations for the other stages of HF are unchanged. Accordingly, practitioners are using both the 2013 ACCF/AHA guideline for the management of HF and the new update.5 To aid the reader’s understanding of the management of stage C HFrEF, the treatment algorithm from the 2013 ACCF/AHA guideline for the management of HF has been updated here to include the 2016 recommendations (FIGURE 1).5,8
New HF Agents
Sacubitril/Valsartan (Entresto): Entresto (sacubitril/valsartan), which was approved in July 2015, is a combination neprilysin inhibitor (sacubitril) and angiotensin II receptor blocker (valsartan). Sacubitril is a prodrug that is converted to LBQ657, its active metabolite, which is an inhibitor of neprilysin. Neprilysin inhibitors work to decrease the degradation of natriuretic peptides. Valsartan inhibits the effect of angiotensin II, as well as angiotensin II-dependent aldosterone release. Sacubitril/valsartan is approved only for use in patients with symptomatic HFrEF despite guideline-directed management.9
The PARADIGM-HF trial compared sacubitril/valsartan in combination with enalapril in patients with HFrEF. In this trial, sacubitril/valsartan reduced the composite endpoint of cardiovascular death by 20% (P <.001). Additionally, sacubitril/valsartan reduced hospitalization due to HF by 20% (P <.001). Finally, symptoms and physical limitations of HF were reduced (P = .001). Patients were aged 18 years or older and had NYHA class II, III, or IV symptoms, with an EF <40%; they also had a B-type natriuretic peptide of at least 150 pg/mL, or at least 100 pg/mL if hospitalized within the past 12 months. Patients already on ACE inhibitor or angiotensin receptor blocker (ARB) therapy had to take a stable dose of ACE inhibitor or ARB equivalent to enalapril 10 mg daily and a beta-blocker for at least 4 weeks prior to the trial. Patients were excluded if they had symptomatic hypotension, systolic blood pressure <100 mmHg at screening or 95 mmHg at randomization, epidermal growth factor receptor (eGFR) <30 mL/minute/1.73 m2 at screening or at randomization, eGFR decrease >25% (this was amended to 35%) between screening and randomization, K+ >5.2 mmol/L at screening (or >5.4 mmol/L at randomization), or a history of angioedema or unacceptable side effects while taking an ACE inhibitor or ARB.7
Sacubitril/valsartan is contraindicated in patients who experienced angioedema while taking an ACE inhibitor or ARB. Sacubitril/valsartan should not be taken concomitantly with an ACE inhibitor, and the ACE inhibitor should be discontinued 36 hours prior to initiation of sacubitril/valsartan. Because aliskiren also inhibits RAAS, the concomitant use of aliskiren and sacubitril/valsartan is contraindicated in patients with diabetes.9
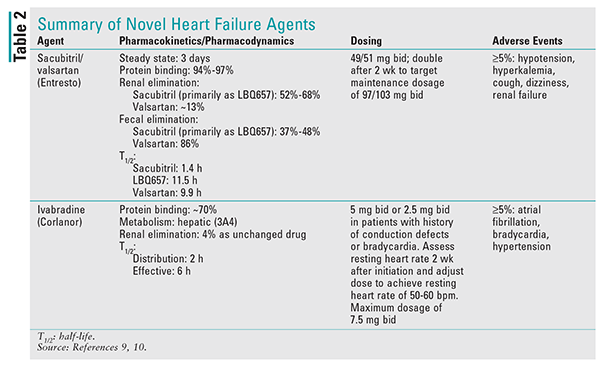
It is important to note that the bioavailability of valsartan in sacubitril/valsartan is higher than in the other available formulations of valsartan. In sacubitril/valsartan, the valsartan component of the 26-mg, 51-mg, and 103-mg strengths is equivalent to the 40-mg, 80-mg, and 160-mg strengths, respectively. See TABLE 2 for additional details on this agent.9
Ivabradine (Corlanor): Approved by the FDA in April 2015, Corlanor (ivabradine) was the first new medication approved for systolic HF in almost a decade.10 Ivabradine is indicated for patients with left ventricular EF (LVEF) of 35% or less, who are in sinus rhythm with resting heart rate 70 bpm or greater, and either are on maximally tolerated beta-blockers or have a contraindication to beta-blocker use.11 Ivabradine has been shown to reduce hospitalization for HF in patients with symptomatic (NYHA class II-III), stable, chronic HFrEF (LVEF 35% or less) who are receiving guideline-based treatment, including a beta-blocker at maximum tolerated dose.8
Ivabradine has a unique mechanism of action compared with other medications approved for HF. To decrease heart rate, ivabradine selectively inhibits the If current by blocking the hyperpolarization-activated cyclic nucleotide–gated channels within the sinoatrial node. This results in delayed depolarization without affecting ventricular repolarization or myocardial contractility. It is important to note that ivabradine can also inhibit the Ih current found in the retina; this causes visual disturbances, specifically phosphenes. These visual disturbances usually occur within 2 months of therapy initiation, and most resolve during treatment or upon discontinuation of the medication.11
SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial) evaluated the effect of ivabradine on cardiovascular outcomes, HF symptoms, and quality of life when added to standard HF therapy in patients with moderate-to-severe HF and LV systolic dysfunction. The addition of ivabradine reduced the risk of the composite endpoint of cardiovascular death or hospital admission for worsening HF symptoms. This effect was driven by a statistically significant reduction in hospital admissions. There was no difference in rate of cardiovascular death between the two groups.12
Patients included in SHIFT were aged 18 years or older and had stable but symptomatic NYHA class II, III, or IV HF in normal sinus rhythm with an LVEF of 35% or less and a resting heart rate of at least 70 bpm. Patients had been admitted to the hospital for worsening HF within the previous 12 months, and they were stable on optimal guideline-directed therapy for at least 4 weeks prior to study initiation. The main exclusion criteria were myocardial infarction within the previous 2 months, ventricular or atrioventricular pacing operative for at least 40% of the day, atrial fibrillation or flutter, and symptomatic hypotension. Also excluded were patients taking nondihydropyridine calcium channel blockers, class I antiarrhythmics, and strong CYP450 3A4 inhibitors. Patients were started on ivabradine 5 mg orally twice daily or placebo along with their other medications for HF. After 2 weeks, the ivabradine dose was adjusted based on resting heart rate.12
Ivabradine is contraindicated in patients with acute decompensated HF, those with severe hepatic impairment, and those who are pacemaker dependent. It also should not be initiated in patients with a blood pressure <90/50 mmHg or a resting heart rate <60 bpm. Ivabradine is primarily metabolized by CYP3A4, and concomitant use with strong inhibitors is contraindicated.13 See TABLE 2 for additional details on this agent.
The Pharmacist’s Role
Pharmacists are in a unique position to assist in HF care. As evidenced here, the use of patient-centered medication therapy can improve patient care in HF. With the addition of two novel agents, the treatment of HF becomes more complex. Although these new agents are promising, cost will be a concern for patients and providers alike. Pharmacists can assist patients and prescribers in appropriate drug selection while considering other factors, such as cost and drug interactions.
REFERENCES
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606-619.
3. Hospital quality initiative: outcome measures. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Accessed October 6, 2017.
4. Medicare Payment Advisory Commission (MedPAC). June 2017 Data Book: Health Care Spending and the Medicare Program. http://medpac.gov/docs/default-source/data-book/jun17_databookentirereport_sec.pdf?sfvrsn=0. Accessed October 6, 2017.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
6. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365-371.
7. McMurray JJ, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
8. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476-1488.
9. Entresto (sacubitril and valsartan) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; November 2017.
10. FDA approves Corlanor® ivabradine to reduce the risk of hospitalization for worsening heart failure in patients with chronic heart failure. www.amgen.com/media/news-releases/2015/04/fda-approves-corlanor-ivabradine-to-reduce-the-risk-of-hospitalization-for-worsening-heart-failure-in-patients-with-chronic-heart-failure. Accessed August 31, 2017.
11. Corlanor (ivabradine) package insert. Thousand Oaks, CA: Amgen Inc; January 2017.
12. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
13. Fala L. Corlanor (ivabradine), first HCN channel blocker, FDA approved for the treatment of patients with heart failure. Am Health Drug Benefits. 2016;9(spec feature):56-59.
To comment on this article, contact rdavidson@uspharmacist.com.






