US Pharm. 2018:43(1)HS-8-HS-11.
ABSTRACT: Cerebral edema is a common consequence of neurologic injuries, and is defined as an abnormal accumulation of fluid within the brain parenchyma. Hyperosmolar therapy is a mainstay of treatment for cerebral edema, creating an osmolar gradient within the blood-brain barrier. Mannitol and hypertonic saline have unique mechanisms of action and adverse effects, but both are efficacious as treatment for cerebral edema. Pharmacists play a vital role to ensure appropriate dosing, monitoring, and treatment goals when using hyperosmolar therapy.
Cerebral edema and elevated intracranial pressure (ICP) are common consequences of neurologic injuries including, but not limited to, intracranial hemorrhage, subarachnoid hemorrhage, ischemic stroke, and traumatic brain injury.1 Cerebral edema is defined as an abnormal accumulation of fluid within the brain parenchyma producing an enlargement of the tissue; it is generally classified as either cytotoxic, interstitial, or vasogenic.2,3 Vasogenic edema is a disruption of the blood-brain barrier leading to the accumulation of protein and fluid in the extracellular space.3 This is the most common form of cerebral edema, and the term cerebral edema will refer specifically to vasogenic edema when referenced throughout this article.
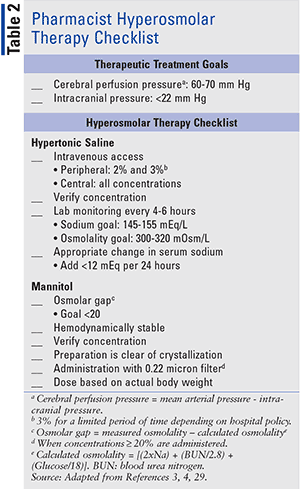
When cerebral edema occurs, it leads to intracranial hypertension (ICH), defined as a sustained ICP greater than 20 mm Hg (normal range: 3–15 mm Hg).1 The treatment goal for cerebral edema is to maintain ICP below 22 mm Hg while maintaining a cerebral perfusion pressure (CPP) between 60 and 70 mm Hg.4,5 CPP is the difference between the mean arterial pressure and ICP.6 ICP measurement is invasive and, thus, cerebral edema can commonly be used as a surrogate for ICH and elevated ICP.
Treatment of Cerebral Edema
The treatment of cerebral edema and ICH includes surgical decompression, head-of-bed elevation, volume resuscitation, hyperosmolar therapy, sedation, hypothermia, and barbiturate coma.7,8 Hyperosmolar therapies, specifically referencing mannitol and hypertonic saline (HTS), create an osmolar gradient, which allows cerebrospinal fluid to move from the cranial space, leading to a decrease in ICP. The focus of this article will be on hyperosmolar therapy with mannitol or hypertonic saline for the treatment of cerebral edema.
Mannitol
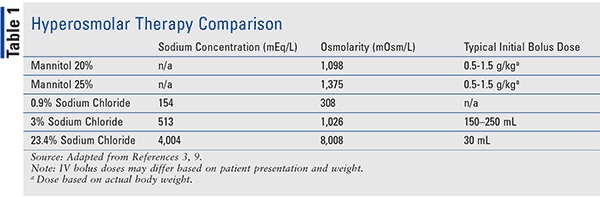
Mannitol is a sugar alcohol (C6H14O6) that decreases water and sodium reabsorption in the renal tubule and has been used for the reduction of ICP or cerebral edema since the 1960s.9 Mannitol lowers ICP through two distinct effects in the brain.10,11 The first, rheological effect, reduces blood viscosity, and promotes plasma expansion and cerebral oxygen delivery. In response, cerebral vasoconstriction occurs due to autoregulation, and cerebral blood volume is decreased. The second effect occurs through creation of an osmotic gradient across the blood-brain barrier, leading to the movement of water from the parenchyma to the intravascular space. Brain tissue volume is decreased and, therefore, ICP is lowered. Mannitol also acts as an osmotic diuretic, leading to free water clearance and an increase in serum osmolality. As a result, water moves from the intracellular to the extracellular space, inducing a prolonged dehydrating effect. Reduction in ICP secondary to mannitol administration is dose-dependent, occurring within 10 to 20 minutes with a peak effect seen between 20 and 60 minutes and lasting between 4 to 6 hours.3 Mannitol is available in concentrations ranging from 5% to 25%, with 20% and 25% concentrations primarily used in acute ICP management. Dosing has historically ranged from 0.18 to 2.5 g/kg/dose (based on actual body weight); however, published literature has found that more significant ICP reductions and sustained responses occur when mannitol is dosed between 0.5 to 1.5g/kg/dose.12-14
Administration of mannitol can occur every 4 to 6 hours after invasive monitoring, such as an external ventricular drain, has been placed.4 Mannitol is typically administered over 20 to 60 minutes; however, faster administration can be used in acute ICP management. When concentrations >20% are administered, use of 0.22 micron in-line filter is required. Mannitol inhibits the resorption of sodium in the renal tubule, leading to a reduction of sodium by up to 13 mEq/L.9 To prevent renal dysfunction, mannitol administration should be avoided when serum osmolality is >320 mOsm/L, but more specifically when the osmolar gap is >20.1,15 Mannitol frequently causes hypotension due to its diuretic effect, making administration in hemodynamically unstable patients less appealing. Acute hypotension is most commonly seen with rapid infusions (<5 minutes) and can be partially mitigated with the use of prolonged infusion rates (15–30 minutes).16
Hypertonic Saline
Hypertonic saline has been utilized for almost a century now, with its first use being documented to decrease brain bulk in 1919 to its use for decreasing elevated ICP and increasing CPP in the late 1980s. The mechanism of action behind this effect has several proposed theories, with the most common involving the creation of an osmotic shift of fluid from the intracellular space to the interstitial and intravascular space. Typical plasma osmolarity ranges between 280 and 295 mOsm/L, while HTS osmolarity can vary from 1026 to 8008 mOsm/L depending on HTS concentration (3%–23.4%), leading to the osmotic fluid shift. Table 1 compares the osmolality of different mannitol and HTS preparations. Further mechanisms include direct vasodilation, increased cardiac output, and potential neurochemical and immune-modulating effects.17
HTS has been studied in various concentrations ranging from the more commonly used 2% and 3% to higher concentrations of 5%, 7.5%, and 23.4%.18 HTS can be administered as a continuous infusion or given as a series of bolus infusions depending on hospital policy. It is important to note that these agents should preferably be administered through a central IV line based on safety issues discussed below, but there is evidence available for the use of 3% HTS through a peripheral IV line.19 HTS has a rapid onset—as quick as 5 minutes—with effects lasting up to 12 hours without any rebound ICP increase.20
The use of HTS is not without concerns for safety and potential adverse effects. Because of its high sodium content, ranging from 513 mEq/L to 4004 mEq/L depending on product concentration (3%–23.4%), there are concerns for electrolyte imbalances (i.e., hypernatremia, hyperchloremia, hypokalemia) and a hyperosmolar state. These specific concerns can cause central pontine myelinolysis (CPM), metabolic acidosis, cardiac depression, and congestive heart failure. Other safety issues that have been reported in published literature include acute kidney injury (AKI), rebound increases in ICP, seizures, altered mentation, coagulopathies, and infusion-rate related hypotension. Intravenous administration of HTS can also cause local effects such as IV infiltration, thrombophlebitis, tissue ischemia, and venous thrombus.19,21-24
Role of the Pharmacist
In addition to monitoring for adverse reactions in patients receiving HTS treatment, an astute pharmacist should observe and double-check numerous other components of therapy. Pharmacists should ensure proper administration technique in terms of correct IV access, rate of infusion, and concentration of HTS products, as well as monitor laboratory values including serum sodium, chloride, potassium, osmolarity, and ICP. Other monitoring parameters, including the use of an electrocardiogram, electroencephalogram, and specific ICP monitors are beyond the scope of this article but important to be aware of.23,24
Treatment Selection
The decision to use salt-based (HTS) or sugar-based (mannitol) osmotherapy has been a long-standing point of contention in hyperosmolar research. Mannitol was the historical treatment of choice; however, HTS has become established as the preferred initial option for the treatment of elevated ICP based on recent data.3,20,25
Clinical determination of treatment should consider the differences in adverse-effect profiles of each agent. Mannitol can cause hypotension secondary to osmotic diuresis, which could potentially be deleterious in patients who are hypotensive or hypovolemic. In contrast, HTS restores intravascular volume and may increase blood pressure as well as lowering ICP. Along with hypotension, mannitol may also be associated with an increased incidence of rebound elevated ICP. This concern is also present with HTS; however, data suggesting this potential side effect is limited. HTS causes hypernatremia, which raises concerns for potential development of CPM with rapid increases in sodium after a hyponatremic state. It is important to note that a majority of the evidence demonstrating the risk of CPM is from animal studies in which supratherapeutic doses were administered. Further evidence has shown that appropriate administration of high sodium loads may mitigate the apparent risk of developing CPM.26-28 Changes in serum sodium should not exceed 12 mEq/L in a 24-hour period to further decrease the risk of CPM.29 Both mannitol and HTS carry the risk of AKI. Patients receiving HTS are at risk for AKI if they have serum sodium >160 mEq/L for a prolonged period. Nephrotoxicity secondary to mannitol is potentially due to osmotic nephrosis, but the risk can likely be avoided by insuring that the osmolar gap is <20 prior to administration. Mannitol increases cerebral blood flow and may be preferable when baseline cerebral hypoperfusion is present.
In addition to varying adverse-effect profiles, potential differences in efficacy may exist between HTS and mannitol. Three meta-analyses comparing the efficacy of HTS and mannitol have shown greater ICP reduction with HTS and a lower incidence of treatment failure.25,30,31 Further studies confirm that HTS reduces the number of treatment failures compared with mannitol.20,32 For ICP elevations >30 mm Hg, HTS provides a more rapid and sustained ICP reduction compared with mannitol.20 Thus, HTS may offer greater efficacy to reduce ICP when other therapies have failed.33,34
Although potential differences in efficacy and outcomes may exist between HTS and mannitol management strategies, further high-quality research is required.4 It is important to note that the majority of research is focused on ICP control as a surrogate marker of efficacy. Although it may be reasonable to establish treatment goals based on this, it does not necessarily correlate with improved patient outcomes. Unfortunately, research in this area is inherently biased due to heterogeneity in both treatment and baseline comorbidities of patients and a lack of standardized dosing regimens.31
Tips for the Pharmacist
Due to the complexity and critical nature of these patients, pharmacists are essential members of the multidisciplinary team. Listed below are tips for pharmacists to help ensure safe and effective therapy delivered in a timely fashion. In a medical emergency, using IV bags rather than vials can save time and resources. It is much more difficult and time-consuming to dispense 100 g of mannitol using 25% vials compared with the premixed 20% bag, which can be administered directly through an IV pump.
When physicians order HTS, always be aware of the patient’s venous access. HTS exceeding 3% should be administered through a central venous catheter owing to the risk of extravasation; however, mannitol may be given via a peripheral IV. Mannitol frequently crystallizes, requiring inspection of the bag or vial for crystals before administration. If crystals are present, this is corrected by warming the solution.
There is no standard dose or concentration for mannitol or hypertonic saline, so orders should always be double checked for accuracy, especially when taking a verbal or telephone order from the physician. Lastly, ensure no hypo-osmotic fluids are currently infusing, including lactated ringers or dextrose 5% in water, which may counteract the hyperosmolar therapy. Table 2 lists hyperosmolar treatment goals and includes a checklist for efficacy and safety for use by pharmacists.
Conclusion
Hyperosmolar therapies present a variety of complex issues and clinical decisions prior to administration. Because of potential side effects and consequences of therapy, pharmacists should insert themselves as key decision makers not only to ensure safe and correct administration, but also appropriate selection of therapy based on the clinical scenario. In order to provide the best outcomes for patients, a consistent and protocolized approach should be taken in the form of order sets and policies with monitoring parameters as well as clear orders and responsibilities for all disciplines. Because of the morbidity and mortality from cerebral edema, it is most important that these patients receive hyperosmolar treatment in a timely and safe fashion.
REFERENCES
1. Fink ME. Osmotherapy for intracranial hypertension: mannitol versus hypertonic saline. Continuum (Minneap, Minn). 2012;3:640-654.
2. Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;3:236-239.
3. Witherspoon B, Ashby NE. The use of mannitol and hypertonic saline therapies in patients with elevated intracranial pressure: a review of the evidence. Nurs Clin North Am. 2017;2:249-260.
4. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma. 2007;24 (suppl1):S14-S20.
5. Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;1:6-15.
6. White H, Venkatesh B. Cerebral perfusion pressure in neurotrauma: a review. Anesth Analg. 2008;3:979-988.
7. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;22:2121-2130.
8. Perez-Barcena J, Llompart-Pou JA, O’Phelan KH. Intracranial pressure monitoring and management of intracranial hypertension. Crit Care Clin. 2014;4:735-750.
9. Hinson HE, Stein D, Sheth KN. Hypertonic saline and mannitol therapy in critical care neurology. J Intensive Care Med. 2013;1:3-11.
10. Smith QR, Rapoport SI. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem. 1986;6:1732-1742.
11. Messeter K, Nordstrom CH, Sundbarg G, et al. Cerebral hemodynamics in patients with acute severe head trauma. J Neurosurg. 1986;64(2):231-237.
12. James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta Neurochir. 1980;51(3-4):161-172.
13. Sorani MD, Morabito D, Rosenthal G. Characterizing the dose-response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma. 2008;4:291-298.
14. Sorani MD, Manley GT. Dose-response relationship of mannitol and intracranial pressure: a metaanalysis. J Neurosurg. 2008;1:80-87.
15. Garcia-Morales EJ, Cariappa R, Parvin CA, Scott MG, Diringer MN. Osmole gap in neurologic-neurosurgical intensive care unit: Its normal value, calculation, and relationship with mannitol serum concentrations. Crit Care Med. 2004;4:986-991.
16. Rosner MJ, Coley I. Cerebral perfusion pressure: a hemodynamic mechanism of mannitol and the postmannitol hemogram. Neurosurgery. 1987;2:147-156.
17. Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009;64(9):990-1003.
18. Lazaridis C, Neyens R, Bodle J, DeSantis SM. High-osmolarity saline in neurocritical care: systematic review and meta-analysis. Crit Care Med. 2013;41(5):1353-1360.
19. Perez CA, Figueroa SA. Complication rates of 3% hypertonic saline infusion through peripheral intravenous access. J Neuroscience Nurs. 2017;49(3):191-195.
20. Alnemari AM, Krafcik BM, Mansour TR, Gaudin D. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurg. 2017;106:509-528.
21. Surani S, Lockwood G, Macias MY, et al. Hypertonic saline in elevated intracranial pressure: past, present, and future. J Intensive Care Med. 2015;30(1):8-12.
22. Froelich M, Ni Q, Wess C, et al. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37(4):1433-1441.
23. Patanwala AE, Amini A, Erstad BL. Use of hypertonic saline injection in trauma. Am J Health Syst Pharm.2010;67(22):1920-1928.
24. Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17(1):117-130.
25. Rickard AC, Smith JE, Newell P, Bailey A, Kehoe A, Mann C. Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emerg Med J. 2014;31(8):679-683.
26. Soupart A, Penninckx R, Namias B, et al. Brain myelinolysis following hypernatremia in rats. J Neuropathol Exp Neurol. 1996;55(1):106-113.
27. White H, Cook D, Venkatesh B. The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesth Analg. 2006;6:1836-1846.
28. Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28(4):1136-1143.
29. Sterns RH, Riggs JE, Schochet SS, Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;24:1535-1542.
30. Mortazavi MM, Romeo AK, Deep A, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;1:210-221.
31. Kamel H, Navi BB, Nakagawa K, et al. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;3:554-559.
32. Burgess S, Abu-Laban RB, Slavik RS, Vu EN, Zed PJ. A systematic review of randomized controlled trials comparing hypertonic sodium solutions and mannitol for traumatic brain injury: implications for emergency department management. Ann Pharmacother. 2016;50(4):291-300.
33. Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144-1451.
34. Horn P, Munch E, Vajkoczy P, et al. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res. 1999;21(8):758-764.
To comment on this article, contact rdavidson@uspharmacist.com.