ABSTRACT: The recent addition of a mixed-features specifier to major depressive disorder has allowed healthcare professionals to uncover a disorder that would have previously remained untreated. Prior to 2013, the mixed-features specifier pertained only to bipolar disorder type 1 diagnoses. Outcomes are far worse than for its unipolar episode comparators. There is a need for a concise tool to recognize mixed features in unipolar depression. Published data indicate the efficacy of monotherapy with atypical antipsychotics as first-line treatment for major depressive disorder with mixed features.
US Pharm. 2017;4211):HS-24-HS-30.
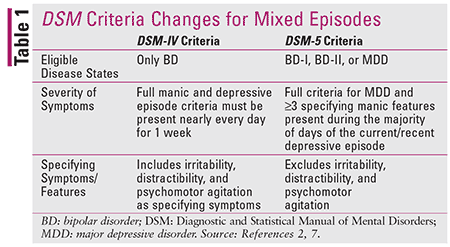
Bipolar disorder (BD) is characterized by distinct episodes of depression and elevated mood (mania or hypomania).1 Historically, manic episodes have been viewed as the hallmark of BD; in fact, the Diagnostic and Statistical Manual of Mental Disorders (DSM) requires a prior manic episode for the diagnosis of BD to be made (Table 1). Further complicating the diagnostic picture is the mixed episode, during which both manic and depressive symptoms are present. Prior to 2013, a mixed episode could be said to occur only in the setting of diagnosed BD; however, when the fifth revision of the DSM (DSM-5) lifted that restriction, it unveiled a previously overlooked, high-risk patient population whose condition appeared to exist outside the realm of formerly accepted diagnoses.1
DSM-5 acknowledges that manic symptoms are not limited to BD; they can coexist with MDD and present a significant treatment challenge.2 This somewhat controversial assertion ushered in a new diagnosis—major depressive disorder with mixed features (MDD-MX)—and was the beginning of a paradigm shift in psychiatry. Pharmacists in all patient care settings are in a frontline position to foster awareness of MDD-MX.
Efforts to promote awareness appear even more imperative when the societal effects of MDD-MX are examined. MDD-MX confers a greater risk of suicidal ideation and the need for inpatient mental health services than is seen in patients with MDD. Not surprisingly, the annual cost to treat a patient with MDD-MX is economically burdensome, with an estimated average cost of $8,402, roughly twice that of MDD.1
These worsened outcomes are undoubtedly a by-product of the frequently prolonged misdiagnosis and resulting treatment resistance associated with MDD-MX.3 It is a diagnostic challenge to differentiate between BD with mixed features, MDD-MX, and unipolar MDD because of the overlapping clinical presentations of these mood disorders. This led the DSM-5 committee to exclude symptoms of irritability, distractibility, and psychomotor agitation from the MDD-MX diagnostic criteria (Table 1). It is postulated that this exclusion could prevent 13% of patients with a true diagnosis of MDD from receiving a false-positive diagnosis of MDD-MX.2-5 However, experts also caution that even nearly perfect diagnostic specificity may still result in misdiagnosis and inadequate treatment of 95% of patients with MDD-MX.4
Before diagnostic criteria were created for MDD-MX, patients presenting as predominantly depressed with fewer than five symptoms of mood elevation were given a diagnosis of MDD because they did not fully meet diagnostic criteria for BD.2 However, patients with undiagnosed MDD-MX who are given monotherapy with antidepressants typically exhibit minimal improvement in depressive symptoms and can experience a worsening of manic symptoms.3
DIAGNOSIS OF MDD-MX
Experts currently recommend asking “every patient, every time” about the presence of manic symptoms if they have a history of depression.2 CUDOS-M is a useful diagnostic tool for clinicians; it is a clinically validated, patient-administered questionnaire that is sensitive to the updated DSM-5 criteria for MDD-MX.6 There are two separate question batteries; the first 18 questions evaluate depressive symptoms and an additional 13 questions detect the presence of underlying mania.
TREATMENT SELECTION
MDD-MX is a newly recognized diagnosis in a stage of clinical discovery. There are few quality randomized, controlled trials (RCTs) currently available to guide treatment selection; as such, clinicians must be cognizant of validity concerns surrounding post-hoc trials that attempt to demonstrate a drug’s efficacy in treating MDD-MX. Subject-inclusion criteria should be critically evaluated before data are extrapolated to patients with MDD-MX; many of the post-hoc analyses either were subgroup analyses conducted in subjects diagnosed with BD or had study-inclusion criteria that differed from DSM-5 diagnostic criteria for MDD-MX.1,2,4,5,7
As previously mentioned, the use of antidepressants, particularly as monotherapy, to treat MDD-MX is highly discouraged. Current practice endorses the use of the following atypical antipsychotics: lurasidone, asenapine, ziprasidone, quetiapine (immediate release or extended release), aripiprazole, and cariprazine as first-line monotherapy (Table 2).2-5,7-18 Because there is a lack of quality supporting evidence for any of the other atypical antipsychotics or mood stabilizers as first-line treatment, this article will exclusively focus on the atypical antipsychotics for which data exist.2,7,8 The side-effect profiles for these agents may be found in Table 3.
Lurasidone
Suppes and colleagues evaluated lurasidone’s efficacy in treating depressive symptoms of MDD-MX by measuring the change in the Montgomery–Åsberg Depression Rating Scale (MADRS) score from baseline to 6 weeks.4 Symptoms of irritability, distractibility, and agitation are not included in DSM-5 diagnostic criteria, and therefore were not included in diagnostic criteria used for the study. However, the study subjects still diverged from the DSM-5 criteria, which requires three or more manic symptoms for diagnosis; in the trial, subjects experiencing only two symptoms were included. Lurasidone decreased MADRS scores for all of the trial subjects, with a robust clinical and statistical significance compared with placebo (P <.001, effect size 0.80). However, for subjects who met DSM-5 criteria, the decreases in MADRS scores in the lurasidone group, although significant, were not as robust (P = .038, effect size 0.50).4 Interestingly, the Goldberg and colleagues study revealed a number needed to treat (NNT) of 4 in subjects with two manic symptoms and an NNT of 28 in subjects who met DSM-5 diagnostic criteria.10
This study laid the groundwork for two post-hoc analyses evaluating lurasidone’s effect on irritability and treatment efficacy over an extended period.5,12 Irritability was determined by a score of 2 on items 5 and 9 of the Young Mania Rating Scale (YMRS). After 6 weeks of treatment, the irritable subjects had a larger decrease in MADRS, YMRS, and Clinical Global Impressions (CGI) scores. The 3-month, open- label, post-hoc extension trial showed sustained improvement in 20.8% of patients who continued their lurasidone treatment once unblinded and in 12.5% of patients switching from placebo to lurasidone.10
While lurasidone’s use in MDD-MX is somewhat confounded by the study’s utilization of alternative diagnostic criteria, both populations showed statistically significant improvement over placebo. However, the NNT increased from 4 to 28 with one additional manic symptom. The robust response in patients with irritable symptoms, although nonspecifying, may be a niche for lurasidone. Overall, lurasidone appears to be a well-tolerated, efficacious treatment option for MDD-MX.10
Asenapine
Like lurasidone, asenapine also has some clinical evidence to suggest its efficacy in MDD-MX, although much of the supportive evidence is extrapolated from studies evaluating subjects diagnosed with BD.11,12 Two such post-hoc studies evaluated asenapine’s use in subjects with BD currently experiencing a manic episode with two or more depressive symptoms.11,12 Asenapine was compared with placebo and olanza-pine for 3 weeks. The primary efficacy endpoint was change in MADRS and YMRS scores from baseline. Beginning at week 1 and sustained at study end, asenapine showed a significant decrease of depressive symptoms independent of initial severity when compared with placebo. One of the studies (98 subjects) found asenapine to be statistically significant in manic-symptom remission versus placebo from day 2 to day 21 (YMRS 12).12 One limitation is the short study duration; 3 weeks is a typical duration for evaluating mania but may be too brief to show clinically meaningful improvement for depression.17 In the other study (960 subjects), there did not appear to be a significant difference in mania remission at study endpoint, with the exception of a subgroup analysis of moderately depressed patients.11 Neither of these trials showed significant separation of asenapine from olanzapine at study end. The trials attempted to find a correlation between treatment efficacy for mild, moderate, and severe depression and the manic symptoms’ response to treatment. They concluded that asenapine had a greater effect on depression remission rates in more severely depressed patients compared with placebo or olanzapine. Furthermore, the remission rate of depression was independent of the manic-symptom remission rates. Caution must be used in drawing any conclusions given the nature of post-hoc analyses, but these results imply that clinical improvement in depressive traits may occur disproportionately to the improvement of mania.
Quetiapine
Quetiapine is FDA approved for the monotherapy or adjunctive treatment of manic, mixed, and depressive states of BD and as adjunctive therapy in treatment-resistant MDD.19 Quetiapine’s efficacy in MDD-MX was evaluated by response and remission rates using CGI scores and concurrent YMRS and MADRS scores for subjects diagnosed with BD-II with mixed features.13 The study’s subjects were considerably ill, with baseline YMRS and MADRS scores of 21.6 and 27.3, respectively, for quetiapine and 20.0 and 29.1 for placebo. Differences in response and remission rates were insignificant for CGI scores (P = .206, P = .132) and YMRS and MADRS scores (P = .19, P = .124) when compared with placebo. For power to have been met, each arm would require 57 subjects rather than the 15 and 12 trial completers. Given the limitations and the plethora of insignificant outcomes, extrapolation of quetiapine’s efficacy in MDD-MX is poorly supported by this study.
Aripiprazole
Aripiprazole’s efficacy in the treatment of manic and mixed episodes in BD is well established; however, there is insufficient evidence to support its efficacy in MDD-MX.15-17 In two RCTs, both manic and mixed BD subjects demonstrated a significant reduction in YMRS and MADRS or CGI-Depression scores, although the clinical significance of the depressive outcomes seems mild at best.15,16 A placebo- controlled RCT evaluated time to rehospitalization for BD mixed- and manic-episode patients over a 26-week poststabilization phase.17 The rates of relapse, when compared with placebo, differed per phenotype; manic states were better protected, which suggests aripiprazole’s marginal effect on depressive symptoms.
Ziprasidone
A study by Patkar and colleagues demonstrated that ziprasidone is another potential treatment option for MDD-MX. The primary efficacy endpoint measured change in MADRS scores over weeks in subjects with MDD and BD-II.14 Ziprasidone showed a significant reduction of 11.4 points in MADRS scores compared with placebo’s reduction of 5.9 at week 6 (P <.05). Half of all subjects receiving ziprasidone had treatment remission (MADRS <9), as contrasted with 18.4% of those receiving placebo (P = .0045). A greater reduction in MADRS scores was seen in BD-II diagnoses than in MDD (P = .036), although there was no significant decrease in manic symptoms between trial arms (P = .23).
It should be noted, however, that the manic symptoms required for study entry included distractibility, irritability, and agitation, creating concerns over the study population’s applicability to DSM-5 criteria. Since one of the only two manic symptoms needed for inclusion was distractibility, which was present in more than half of the population (58%), these findings should be applied to the treatment of MDD-MX with caution.14
Cariprazine
Cariprazine is an atypical antipsychotic indicated for the acute treatment of manic or mixed BD-I episodes.20 An initial exploratory 8-week trial of 233 subjects failed to show an improvement in depressed-state BD episodes with dosages of cariprazine up to 3 mg/day.21 In a second attempt to gain approval, an 8-week RCT with 600 subjects was conducted.18 This trial found 1.5 mg of cariprazine to have a significant response (≥50% reduction in MADRS score) and remission (MADRS score of ≤10) of depressive symptoms compared with placebo (odds ratio [OR] = 2.10, P = .002; and OR = 2.38, P = .002, respectively). One explanation for the difference in results could be a third active metabolite (didesmethyl cariprazine, DDCAR) that was unaccounted for in the exploratory study.19 DDCAR can take 6 to 8 weeks to reach peak concentration and likewise necessitates that trial dosages be evaluated individually. However, the inconsistency of hypomania/depressive symptomatology in trial subjects limits the applicability of cariprazine to MDD-MX based on published literature. Additional studies are needed to evaluate cariprazine’s efficacy in treating MDD-MX.
To summarize, the evidence supports the use of lurasidone for treatment of MDD-MX. The following atypical antipsychotics, listed in descending order based on strength of currently available evidence, may be used with caution: ziprasidone, asenapine, cariprazine, aripiprazole, and quetiapine.
ROLE OF THE PHARMACIST
Pharmacists in all practice settings are uniquely positioned to educate patients about MDD-MX. Patients receiving an antidepressant, particularly for the first time, should be encouraged to contact their healthcare provider if they experience a persistently elevated mood, decreased need for sleep, racing thoughts, or increased participation in high-risk activities, as these could be symptoms of MDD-MX.7 Most patients have not heard of this diagnosis and, accordingly, will not correlate the development of these symptoms to their antidepressant. Additionally, MDD-MX should be considered in patients with a presenting diagnosis of unipolar MDD who do not exhibit a satisfactory therapeutic response to monotherapy antidepressants. Patients should be counseled to monitor and report their symptomatic response to antidepressants, or lack thereof, to their healthcare provider.
CONCLUSION
MDD-MX is a frequently misdiagnosed mood disorder that is associated with high rates of suicidality if inadequately treated. The presentation of MDD-MX mimics that of unipolar MDD; unfortunately, commonly prescribed antidepressants can exacerbate the subthreshold manic symptoms of MDD-MX. The pharmacist’s preemptive and conscientious counseling is integral to ensuring that patients receive adequate treatment.
REFERENCES
1. McIntyre R, Ng-Mak D, Chien-Chia C, et al. Major depressive disorder with subthreshold hypomanic (mixed) features: a real-world assessment of treatment patterns and economic burden. J Affect Disord. 2016;210(2017):332-337.
2. Stahl S. Mixed-up about how to diagnose and treat mixed features in major depressive episodes. CNS Spectr. 2017;22(2):111-115.
3. Faedda G, Marangoni C. What is the role of conventional antidepressants in the treatment of major depressive episodes with mixed features specifier? CNS Spectr. 2017;22(2):120-125.
4. Suppes R, Silva J, Cucchiaro Y, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173(4):400-407.
5. Swann A, Fava M, Tsai J, et al. Lurasidone for major depressive disorder with mixed features and irritability: A post-hoc analysis. CNS Spectr. 2017;22(2):228-235.
6. Zimmerman M, Chelminski I, Young D, et al. A clinically useful self-report measure of the DSM-5 mixed features specifier of major depressive disorder. J Affect Disord. 2014;168:357-362.
7. Stahl S, Morrissette D, Faedda G, et al. Guidelines for the recognition and management of mixed depression. CNS Spectr. 2017;22(2):203-219.
8. Benazzi F. Family history validation of a definition of mixed depression. Compr Psychiatry. 2005;46:159-166.
9. Frye M, Helleman G, McElroy S, et al. Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry. 2009;166:164-172.
10. Goldberg J, Ng-Mak D, Siu C, et al. Remission and recovery associated with lurasidone in the treatment of major depressive disorder with subthreshold hypomanic symptoms (mixed features): post-hoc analysis of a randomized, placebo-controlled study with longer-term extension. CNS Spectr. 2017;22(2):220-227.
11. McIntyre R, Tohen M, Berk M, et al. DSM-5 mixed specifier for manic episodes: evaluating the effect of depressive features on severity and treatment outcome using asenapine clinical trial data. J Affect Disord. 2013;150:378-383.
12. Berk M, Tiller JW, Zhao J, et al. Effects of asenapine in bipolar I patients meeting proxy criteria for moderate-to-severe mixed major depressive episodes: a post hoc analysis. J Clin Psychiatry. 2015;76:28-734.
13. Suppes T, Ketter T, Gwizdowski I, et al. First controlled treatment trial of bipolar II hypomania with mixed symptoms: quetiapine versus placebo. J Affect Disord. 2013;150:37-43.
14. Patkar A, Gilmer W, Pae C, et al. A 6 week randomized double-blind placebo-controlled trial of ziprasidone for the acute depressive mixed state. PLoS One. 2012;7(4):e34757.
15. Keck P, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160:1651-1658.
16. Sachs G, Sanchez R, Marcus R, et al. Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol. 2006;20:536-546.
17. Keck P, Calabrese J, McQuade R, et al. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry. 2006;67:626-637.
18. Durgam S, Earley W, Lipschitz A, et al. An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry. 2016;173:271-281.
19. Seroquel XR (quetiapine) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2009.
20. Vraylar (cariprazine) package insert. Irvine, CA: Allergan USA, Inc; 2016.
21. Citrome L. Cariprazine in bipolar disorder: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013;30(2):102-113.
To comment on this article, contact rdavidson@uspharmacist.com.