US Pharm. 2017;42(11):HS18-HS23.
ABSTRACT: While obesity and other risk factors for metabolic syndrome, such as type 2 diabetes, are increasing in prevalence in the general population, patients with serious mental illness (SMI) are reported to experience substantially greater cardiovascular-related mortality compared with age-matched peers without SMI. Causes of cardiometabolic syndrome include not only the adverse metabolic side effects associated with antipsychotic agents, but also poor lifestyle choices and reduced access to medical care. Pharmacists must play a proactive role in exploring opportunities to assist patients with SMI in both optimizing medication management and incorporating lifestyle modifications to improve health outcomes.
Metabolic syndrome has become increasingly prevalent in the United States and is now considered relatively common in the general population. It is frequently implicated as a cause of cardiovascular (CV) complications. Elevated triglycerides (TG), low HDL cholesterol, high blood pressure (BP), increased blood glucose (BG), and abdominal adiposity associated with weight gain are specific elements of this condition.1-3
Metabolic Syndrome in Serious Mental Illness
It has long been recognized that, for numerous reasons, patients with serious mental illness (SMI) have a shorter life expectancy than their peers in the community who do not have SMI. This life-expectancy gap has been reported to be as much as 25% shorter, and the premature mortality is often associated with CV disease.1 Although anyone can develop metabolic syndrome, persons with SMI are likelier to develop this condition because of high-risk lifestyle conditions and antipsychotic-induced CV side effects. Side effects associated with drug-induced weight gain and the worsening of other metabolic parameters, such as dyslipidemia, vary among the different antipsychotic agents and often spark debate over the magnitude of the potential adverse impact (TABLE 1).1
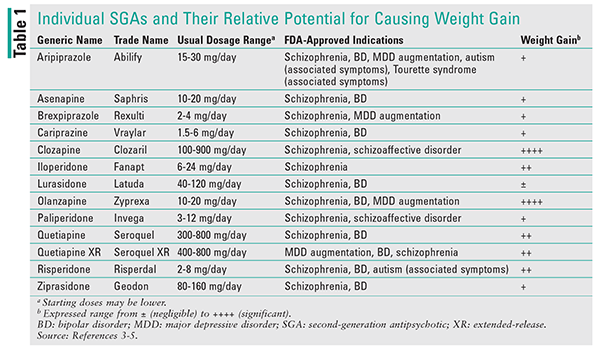
Rummel-Kluge and colleagues conducted a meta-analysis that examined outcomes for three metabolic symptoms: changes in weight, cholesterol, and BG.4 Weight gain was considered to be a stronger metabolic marker, as it is subject to less fluctuation than other markers that can be challenged because of diurnal fluctuations (e.g., fasting vs. fed state). Although all second-generation antipsychotics (SGAs) have been implicated in weight gain, olanzapine and clozapine were associated with the greatest impact on elevation of all three markers, whereas quetiapine and risperidone were associated with an intermediate impact and aripiprazole and ziprasidone had the lowest impact (least effect).4,5
Quetiapine has demonstrated slightly greater elevations in cholesterol compared with other intermediate-impact SGAs, and there are no statistical differences between quetiapine and clozapine or olanzapine. The enhanced ability of quetiapine to alter cholesterol and TG is supported by CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness).6 Despite the challenges of reliably confirming drug-induced changes in BG, clozapine and olanzapine produced significant increases in BG compared with all other SGAs.
Side effects reported in these studies were based on the relative risk associated with usual doses within the recommended therapeutic range. Longer-term studies (>38 weeks) generally showed more weight gain than shorter studies (<12 weeks), and the rate of weight gain was typically rapid during initiation until a peak plateau was achieved, albeit at different rates for different agents (e.g., clozapine has been reported to plateau at 42-46 months compared with olanzapine at 4-9 months). It should also be noted that risk varies based on patient-specific factors and that the magnitude of the change is not always predictable, especially with intermediate-impact agents. Until the relatively newer agents such as lurasidone, cariprazine, and brexpiprazole have had sufficient length of exposure in the general population, aripiprazole and ziprasidone remain the agents least likely to contribute to the development of metabolic syndrome.4,5
Weight gain and obesity are often considered the main metabolic challenges in mental health; however, according to the International Diabetes Federation, criteria for metabolic syndrome include central obesity plus any two of the following: elevated TG, reduced HDL cholesterol, elevated BP, and elevated fasting plasma glucose or previously diagnosed type 2 diabetes mellitus (T2DM).7-9 It is important to remember that although overall BMI is important, central adiposity (waist circumference; i.e., distribution of fat) is the defining characteristic leading to higher risk.8
Hyperlipidemia
Although little is known about the mechanism of antipsychotic-induced metabolic changes, SGAs have been associated with a higher risk of metabolic syndrome. Among SGAs there are notably greater offenders, such as olanzapine and clozapine, and lesser offenders, such as asenapine, lurasidone, iloperidone, and ziprasidone. Poor diet and a lack of beneficial physical activity also contribute to the risk of developing hyperlipidemia.
If nonpharmacologic treatment interventions fail to demonstrate improvement, pharmacologic selections must be based on the patient’s risk factors for developing arteriosclerotic CV disease (ASCVD), which is defined as a history of one or more of the following comorbidities: myocardial infarction/acute coronary syndromes, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, and peripheral artery disease presumed to be of atherosclerotic origin.10
Patients should be encouraged to improve modifiable risk factors, which include smoking cessation and control of cholesterol, BG, and BP. However, the nonmodifiable factors age, sex, and race are also used to calculate the final ASCVD risk score.11 The 2013 AHA guidelines recommend the use of either of the two available high-intensity statins—atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg—in patients aged 75 years or younger with clinical ASCVD or in any patient with LDL 190 mg/dL or greater or 189 mg/dL or less with T2DM. Prescribers treating SMI patients with comorbid hyperlipidemia may choose to avoid high-intensity statins because of the potential for muscle cramps—which could discourage further treatment acceptance—or fear of the potential psychiatric side effects of memory impairment and aggression, which have been suggested but not definitively proven or refuted to date.
In a 2015 case series, Cham and colleagues found that cholesterol-lowering therapy was associated with behavioral changes, including aggression (which resolved upon treatment discontinuation and recurred upon rechallenge, suggesting a causative relationship).12 It should be noted that some patients in this series had underlying psychiatric conditions, indicating that psychiatric illnesses may have been exacerbated. Leppien and colleagues found no significant relationship between statin therapy and agitation, but they found a significant relationship between total cholesterol level and aggression, concluding that psychiatric inpatients with lower total cholesterol levels may be at increased risk for loss of behavioral control.13 Sahebzamani and colleagues, however, reported conflicting relationships between LDL and total cholesterol values and observed behavioral changes such as self-reported ratings of depression, aggression, cynicism, and hostility.14 Therefore, further research into the use of statins in SMI patients is needed to confirm or refute such an association.
The debate over whether statins can cause memory deficits intensified following FDA-mandated changes to drug labels in February 2012 that warned about memory problems associated with short-term statin use.15 Later studies not only found a lack of connection between short-term statin use and memory loss, but also identified a potential association between longer-term statin use and protection against dementia in patients without baseline cognitive dysfunction.16 Despite the controversy, extra care in monitoring cognitive function is recommended in the psychiatric patient population; if memory loss is recognized, patients should be advised that this effect will resolve when the statin is discontinued.
Hyperglycemia
In early 2017, the American College of Physicians (ACP) released new guidelines on T2DM that updated those from 2012, which were developed to provide clinical recommendations on oral pharmacologic treatment of T2DM in all adults regardless of the mental-health condition. Although the general recommendations in the two ACP guidelines are similar, they are further supported by the release of the American Diabetes Association’s 2017 Standards of Care recommendations, which suggest the use of metformin to prevent T2DM in patients with prediabetes (especially those with BMI 35 kg/m2 and those aged <60 years) and patients with rising A1C values despite attempts to correct A1C through lifestyle interventions.17,18
Metformin is effective for reducing BG concentrations, is associated with weight loss and fewer hypoglycemic episodes, and is less expensive than other T2DM medications. However, debate surrounding restrictions in patients with renal impairment and the potential association with lactic acidosis may have limited the use of this antidiabetic agent. To expand access to metformin, the FDA reduced restrictions on its use in patients with moderate chronic kidney disease (CKD). This recommendation, published in April 2016, was based on data showing that these patients are not at significantly elevated risk for lactic acidosis. The contraindication now applies only to patients with severe CKD (<30 mL/min/1.73 m2).19
The use of metformin to prevent metabolic syndrome, weight gain, and glycemic shifts in patients with mental illness is an increasingly intensifying debate. Studies have explored the potential role of metformin in preventing diabetes in nondiabetic patients who are prescribed antipsychotic agents. Wu and colleagues concluded that lifestyle interventions and metformin, both alone and in combination, were effective for weight loss and increased insulin sensitivity; lifestyle interventions plus metformin had the greatest effect on weight loss; and metformin alone was more effective than lifestyle interventions alone in increasing insulin sensitivity and reversing weight gain in patients with schizophrenia whose significant weight gain was caused by antipsychotic medications.20 The routine use of metformin in nondiabetic patients for prevention of metabolic syndrome has not been officially recommended.
In patients with SMI and diabetes, the goal of therapy is to reduce overall long-term complications and, in the short term, to avoid hypoglycemia, which may present as worsening cognitive status or emerging psychiatric symptoms. Less stringent A1C goals of less than 8% may be appropriate for SMI patients who have a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing T2DM in which the therapy goal is difficult to achieve despite diabetes self-management and appropriate pharmacologic interventions.18 Diabetes management requires significant, ongoing education and careful monitoring in all patients.
Hypertension
Antipsychotic agents are commonly associated with orthostatic hypotension and BP-lowering effects resulting from receptor antagonism primarily on alpha-1 adrenergic receptors, although other neurotransmitter actions also have been associated with these effects.3 Patients with SMI who also exhibit elevated BP would likely present with even higher BP readings in the absence of the prescribed antipsychotics. The medical management of hypertension in SMI patients may be complicated by numerous potentially significant drug interactions in those taking additional mood stabilizers such as lithium, which could result in neurotoxicity or dangerously elevated serum lithium concentrations.3 Interventions such as increased physical activity, careful evaluation and control of sodium intake, incorporation of the DASH (Dietary Approaches to Stop Hypertension) diet (which is largely plant-based and low in fat), and smoking cessation are critical elements in establishing a healthier lifestyle. The 2013 Eighth Joint National Committee guidelines recommend that the BP goal for patients with co-occurring T2DM or CKD be less than 140/90 mmHg, but may be more loosely restricted to no more than 150/90 mmHg in patients older than 60 years with medical comorbidity, regardless of mental-health status.21
Smoking Cessation
Patients with mental illness are reported to have a higher rate of smoking than the general population and to smoke twice as many cigarettes that are inhaled more deeply compared with smokers without mental illness.22,23 In patients with certain serious chronic mental-health disorders, such as schizophrenia, long-term nicotine replacement may improve long-term abstinence rates, although further studies are needed. The elevated smoking rate has been hypothesized to be an attempt to self-medicate, and research has supported the beneficial pharmacologic actions of nicotine in interacting with the glutaminergic and dopaminergic neurotransmitter systems. Attempts have been made to develop selective alpha-7 nicotinic agonists for the treatment of impaired sensory gating, negative symptoms, and cognitive dysfunction; however, formal testing has not yet been conducted.24
It is necessary to perform a careful medication review before initiation of a smoking-cessation plan to ensure that the return to baseline hepatic metabolism does not affect serum concentrations of the medications the patient is currently taking. Medications extensively metabolized in the liver, mainly by CYP1A2, are strongly influenced by the polycyclic aromatic hydrocarbons (products of incomplete combustion) in tobacco smoke that induce this enzyme. Therefore, smokers who take certain medications, such as clozapine or olanzapine, are usually receiving higher doses of these medications but have lower plasma concentrations compared with nonsmoking peers. The pharmacokinetic consequences of the restoration and stabilization of 1A2 enzyme activity resulting from smoking cessation are increased serum levels of the substrate drug and potentially increased adverse effects.25
Diet and Exercise
Establishing a routine of regular exercise promoting cardiorespiratory fitness has been shown to reduce the risks associated with all-cause mortality in patients with cardiometabolic syndrome. This lifestyle intervention, along with the incorporation of a healthful diet, has had a more favorable effect on reducing the development of T2DM compared with oral antihyperglycemic agents alone.26
The Pharmacist’s Role
Key to the improving the health of patients with SMI is an effective integration of psychiatric and physical medical care. The pharmacist serves as liaison and as manager of the delicate and complicated balance of medications used throughout the psychiatric and medical-management spectrums. Pharmacists can also play a critical role in identifying risk factors for metabolic syndrome and recommending interventions to prevent further worsening of metabolic indices, thereby reducing overall medical complications in patients who have developed metabolic syndrome. Although psychiatrists may accept responsibility for detecting or monitoring metabolic syndrome, primary-care providers will likely be responsible for treating these conditions if this care continuum is available and accessible. Because these patients are often burdened with significant health disparities, they often receive lower-quality care and experience worse outcomes than non-SMI peers in the community.
Pharmacists must work collaboratively with healthcare personnel involved in the treatment of patients with mental illness in order to improve access; they also must continue to explore methods to better engage SMI patients in self-directed efforts to modify lifestyle and other risk factors.1 Offering suggestions for incorporating healthful eating into daily routines and helping set realistic goals for increased daily physical activity are important coaching tools to encourage patients who are learning to make lifestyle modifications. Pharmacists can evaluate antipsychotic regimens for specific drugs with a potentially negative metabolic impact and provide alternative recommendations to mitigate drug-induced sedation, which may pose yet another barrier to initiation of an exercise regimen.
Conclusion
The CV risks associated with underlying mental illness and the potential for antipsychotic agents to intensify metabolic dysfunction underscore the importance of careful initial selection of medication used in this patient population. The patient’s clinical status, demographics, and physical condition, along with continual screening to ensure early detection of metabolic abnormalities, are critical to achieving improved health, quality of life, and length of life in patients with SMI. As the impact of genetic factors on disease is explored, claims that the newer SGAs may offer a more metabolically benign option to treat psychiatric symptoms continue to be investigated, allowing for the selection of a lower-risk medication with careful routine monitoring for early detection of metabolic abnormalities. Failure to optimize medication management in patients diagnosed with SMI will prevent successful interdisciplinary efforts to improve the current rates of morbidity and mortality in this population.
REFERENCES
1. Ganguli R, Strassnig M. Prevention of metabolic syndrome in serious mental illness. Psychiatr Clin North Am. 2011;34:109-125.
2. Monteleone P, Martiadis V, Maj M. Management of schizophrenia with obesity, metabolic, and endocrinological disorders. Psychiatr Clin North Am. 2009;32:775-794.
3. Clinical Pharmacology (online database). Tampa, FL: Gold Standard, Inc; 2013.
4. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
5. Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PloS One. 2014;9:e94112.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19-32.
7. Riordan HJ, Antonini P, Murphy MF. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia: risk factors, monitoring, and healthcare implications. Am Health Drug Benefits. 2011;4:292-302.
8. International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. www.idf.org/our-activities/advocacy-awareness/resources-and-tools/60:idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html. Accessed July 19, 2017.
9. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
10. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
11. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
12. Cham S, Koslik HJ, Golomb BA. Mood, personality, and behavior changes during treatment with statins: a case series. Drug Saf Case Rep. 2016;3:1.
13. Leppien E, Mulcahy KB, Demler TL, et al. Effects of statins and cholesterol on patient aggression: is there a connection? Innov Clin Neurosci. 2017;14 [in press].
14. Sahebzamani FM, D’Aoust RF, Friedrich D, et al. Relationship among low cholesterol levels, depressive symptoms, aggression, hostility, and cynicism. J Clin Lipidol. 2013;7:208-216.
15. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 4, 2017.
16. Swiger KJ, Manalac RJ, Blumenthal RS, et al. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213-1221.
17. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:279-290.
18. American Diabetes Association. Prevention or delay of type 2 diabetes. Diabetes Care. 2017;40(suppl 1):S44-S47.
19. FDA. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed July 19, 2017.
20. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185-193.
21. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
22. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606-2610.
23. Gelenberg AJ, de Leon J, Evins AE, et al. Smoking cessation in patients with psychiatric disorders. Prim Care Companion J Clin Psychiatry. 2008;10:52-58.
24. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021-1034.
25. Cole ML, Trigoboff E, Demler TL, Opler LA. Impact of smoking cessation on psychiatric inpatients treated with clozapine or olanzapine. J Psychiatr Pract. 2010;16:75-81.
26. Gill H, Mugo M, Whaley-Connell A, et al. The key role of insulin resistance in the cardiometabolic syndrome. Am J Med Sci. 2005;330:290-294.
To comment on this article, contact rdavidson@uspharmacist.com.






