US Pharm. 2017;42(12)15-19.
ABSTRACT: Opioid-induced constipation (OIC) is a common adverse effect experienced by many patients on opioid therapy for chronic pain. Inadequate treatment of OIC is a barrier to the management of chronic pain and leads to a poorer quality of life. Nonpharmacologic treatment includes dietary and lifestyle changes. OTC medications include stimulant laxatives, stool softeners, and enemas. Prescription medications include lubiprostrone, methylnaltrexone, naloxegol, and naldemedine. Pharmacists can take a proactive approach in helping patients prevent and manage OIC by making recommendations on therapy selection and dose adjustments and by counseling on the side effects of their medication.
Although opioid prescribing has declined slightly over the last 5 years in the United States—from a peak of 255 million prescriptions in 2012 to 215 million in 2016—opioids remain the most commonly used major pain-relieving medications for chronic noncancer pain.1,2 Opioids are associated with a spectrum of adverse effects (AEs), including sedation, respiratory depression, tolerance, nausea, vomiting, abdominal pain, and constipation. Gastrointestinal (GI) problems are the most common AEs associated with opioid use; however, unlike other GI AEs, which improve over time, opioid-induced constipation (OIC) is persistent.2 OIC is caused by activation of the enteric mu-opioid receptors, which leads to reduced gastric, biliary, pancreatic, and intestinal secretions, increased absorption of water from bowel contents, and decreased gastric motility.3
OIC in patients treated for noncancer pain has a prevalence of approximately 40% to 95%, and it is more likely to develop with longer duration of opioid use.2-4 OIC usually impairs the patient’s ability to perform activities of daily living and causes more missed work time, less work productivity, and lower levels of overall health and well-being.5,6 Treatment satisfaction declines with the presence of OIC, and many patients discontinue opioid therapy for this reason even if it provides pain relief.2 Therefore, the failure to anticipate and prevent OIC or to adequately treat it once it develops is a major barrier to effective management of chronic pain.
Although the patient’s experience of non-OIC and OIC may be the same, the physiological causes of these conditions differ greatly. Non-OIC may be due to dietary choices, dehydration, or inactivity, whereas OIC is due to the previously discussed physiological changes opioids cause in the GI tract.
DIAGNOSIS
The need to distinguish between constipation and OIC led to the addition of OIC to the 2016 Rome IV criteria as a diagnosis separate from constipation within bowel disorders.7 For OIC, the Rome criteria specify new or worsening symptoms of constipation when initiating, changing, or increasing opioid therapy that must include two or more of the following: straining during more than one-fourth of defecations; lumpy or hard stools for more than 25% of defecations; sensation of incomplete evacuation for more than 25% of defecations; sensation of anorectal obstruction or blockage for more than 25% of defecations; manual maneuvers to facilitate more than 25% of defecations (e.g., digital evacuation, pelvic-floor support); and fewer than three spontaneous bowel movements per week, with loose stools rarely occurring without laxative use.7
More important than the diagnostic criteria is the fact that patients may define both constipation and OIC as hard, difficult-to-pass stools.4 Pharmacists are well positioned to counsel patients on this AE and to discuss appropriate nonpharmacologic and pharmacologic interventions.
TREATMENT
Dietary and Lifestyle Changes
Adjustments in dietary and lifestyle habits can effect notable improvements in OIC or constipation from any cause. To help improve constipation, current guidelines recommend daily ingestion of 25 to 30 g of dietary soluble fiber; adequate fluid ingestion (1.5-2 L daily); regular aerobic exercise (adjusted to individual physical fitness and preferences); balanced diet; regular meal pattern; and avoidance of heavy meals, fat, insoluble fiber, and flatulent foods.7 Although these measures should be recommended to patients during pharmacy consultation for opioid initiation, it is unlikely that dietary and lifestyle changes alone will prevent or treat OIC.4
OTC Products
General consensus is that, in addition to lifestyle changes, stimulant laxatives should be initiated with opioid treatment to prevent OIC.4 On the whole, stimulant laxatives, stool softeners, and enemas are equally recommended based on patient preference and efficacy.3 Because these products are available OTC, pharmacists should be well versed in their appropriate use and place in OIC therapy. The most common regimens involve the combination of a stimulant laxative, such as bisacodyl or senna, and a stool softener. There are three types of stool softeners: surfactants, lubricants, and osmotics. Surfactants such as docusate sodium are emulsifiers that facilitate the admixture of fat and water in the feces. Lubricants such as mineral oil delay absorption of water from stools in the colon, thus softening the feces. Osmotics draw water into the colon to hydrate the stools.4 Bulk-forming laxatives such as psyllium should be avoided because they increase stool bulk and distend the colon, which can worsen abdominal pain and bowel obstruction when opioids prevent peristalsis.4 Information regarding dosing, onset of action, and side effects of OTC laxatives and stool softeners is given in TABLE 1.8
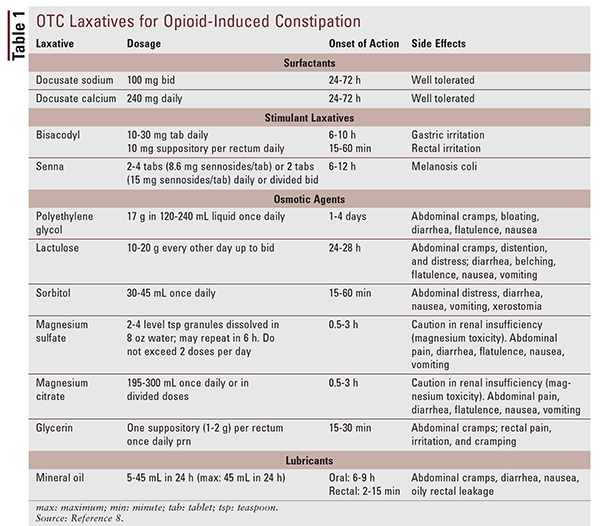
Prescription Products
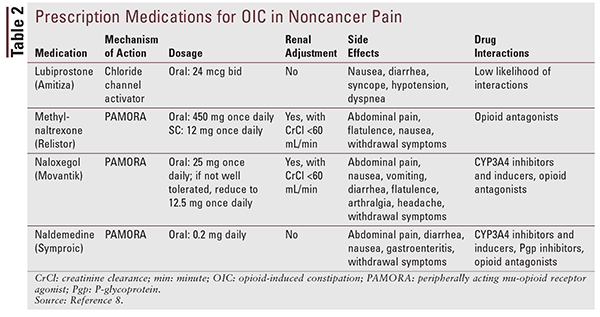
When the combination of diet, lifestyle, and OTC laxatives and stool softeners is insufficient to relieve OIC, most clinicians and patients turn to prescription medications. Four prescription products are approved for OIC in adults with chronic noncancer pain: lubiprostone, methylnaltrexone, naloxegol, and naldemedine.
Lubiprostone: Approved in 2013, lubiprostone (Amitiza) is a chloride channel activator that works in the GI tract to increase intestinal-fluid secretion and improve fecal transit.9,10 The recommended dosage of lubiprostone is 24 mcg twice daily with food and water. Lubiprostone is also available in 8-mcg capsules for irritable bowel syndrome with constipation; when dispensing lubiprostone, the pharmacist should ensure that the correct dose has been prescribed. When lubiprostone is used for OIC, nausea and diarrhea are the most common side effects.10 Dyspnea, syncope, and hypotension may occur after the first dose is taken, especially in patients on concomitant antihypertensive medications. These effects generally resolve; however, they may recur with subsequent dosing.9 Mechanical GI bowel obstruction is a contraindication to lubiprostone, and patients should be evaluated prior to starting the medication. No dose adjustment is needed in renal impairment, but adjustment should be made in hepatic impairment. CYP450 isoenzymes are not involved in lubiprostone metabolism; therefore, the likelihood of drug-drug interactions is low.9 Lubiprostone is well tolerated and effective with long-term use of up to 13 months.10 The efficacy of lubiprostone has not been established in patients taking diphenylheptane opioids (e.g., methadone); in fact, methadone appears to prevent the benefits offered by lubiprostone.11
Overview of Peripherally Acting Mu-Opioid Receptor Agonists (PAMORAs): The other three medications approved for OIC in noncancer pain—methylnaltrexone, naloxegol, and naldemedine—are in the PAMORA class.12-14 PAMORAs preferentially block mu-opioid receptors in the periphery and do not interfere with the analgesic affects of opiates on the mu receptor in the central nervous system. However, a low risk of opioid withdrawal exists, so patients started on a PAMORA should be monitored for withdrawal symptoms such as hyperhidrosis, rhinorrhea, anxiety, and chills. Opioid antagonists such as naloxone and naltrexone should not be used in conjunction with PAMORAs because of the potential for additive effects and increased risk of withdrawal. To be considered a candidate for these medications, a patient must be on opioid therapy for at least 4 weeks. Laxatives should be discontinued prior to PAMORA initiation; if adequate relief of constipation does not occur within 3 days, then laxatives may be reintroduced. When opioid therapy is discontinued, the PAMORA should also be discontinued.12-14 Although they are in the same class, each PAMORA has unique characteristics, as discussed below.
Methylnaltrexone: Methylnaltrexone bromide (Relistor) is unique among the PAMORAs in that it is available in both oral and SC-injection formulations. The injectable formulation was approved in 2008, and the oral formulation gained approval in 2016. The SC injection has an additional indication for treatment of OIC in adults with advanced illness who are receiving palliative care when response to laxative therapy has been insufficient.12 The oral formulation of methylnaltrexone bromide is available in 150-mg tablets. The recommended dosage is 450 mg once daily in the morning on an empty stomach at least 30 minutes before the first meal of the day. The injectable formulation is dosed based on body weight. Patients should be advised to remain near a toilet once the dose is taken.12 Common side effects of this medication include abdominal pain, flatulence, and nausea.12
Methylnaltrexone is contraindicated in patients with mechanical GI bowel obstruction and in those who are at increased risk for recurrent obstruction.12 In renal or hepatic impairment, the dose of oral or injectable methylnaltrexone should be adjusted.12 Oral methylnaltrexone was approved based on results of a randomized, double-blind, placebo-controlled study comparing 4-week treatment with methylnaltrexone 150 mg, 300 mg, or 450 mg orally once daily versus placebo.15 Oral methylnaltrexone 450 mg was particularly effective and well tolerated for OIC in patients with chronic noncancer pain.15
Naloxegol: In 2014, naloxegol (Movantik) was approved for treatment of OIC in adults with noncancer pain, including those with chronic pain related to prior cancer or its treatment who do not require frequent opioid dose escalation. If it is not well tolerated, the recommended dosage (25 mg orally daily) may be reduced to 12.5 mg. Naloxegol may be crushed, if necessary, and may be given via nasogastric tube.13 It should be taken on an empty stomach 1 hour before or 2 hours after the first meal of the day. Renal adjustment is necessary, and naloxegol is not recommended in severe hepatic impairment.13 Grapefruit and grapefruit juice should be avoided owing to a possible increase in naloxegol concentration.13
Naloxegol has the same contraindication as me-thylnaltrexone with regard to GI obstruction; however, it is also contraindicated with concomitant use of strong CYP3A4 inhibitors such as clarithromycin and ketoconazole, as these medications may increase the concentration of naloxegol and the risk of AEs.13 The naloxegol dosage should be decreased with concomitant use of moderate CYP3A4 inhibitors such as diltiazem, erythromycin, and verapamil.13 CYP3A4 inducers such as rifampin may make naloxegol less effective.13 The most common side effects of naloxegol are abdominal pain, nausea, vomiting, diarrhea, flatulence, arthralgia, and headache.13 In clinical studies, patients receiving naloxegol 25 mg were more likely to experience AEs, primarily GI in nature, compared with patients receiving either naloxegol 12.5 mg or placebo.16,17 Studies have shown that naloxegol improves quality of life, is generally safe and well tolerated, and offers good response without reducing opioid-mediated analgesia.16-19 Reduced reliance on other laxatives was also seen in studies because naloxegol alone is usually sufficient for relief of OIC.18 A phase II, double-blind, randomized, placebo-controlled study found that naloxegol improved physical and social functioning, as well as mental health and vitality.17
Naldemedine: Approved in March 2017, naldemedine (Symproic) is the newest PAMORA to receive FDA approval for treatment of OIC in adults with chronic noncancer pain. Naldemedine is structurally related to naltrexone, making it a Schedule II controlled substance. Shionogi Inc., the manufacturer of naldemedine, has requested that the FDA remove the controlled-substance classification; the FDA’s decision is pending. The recommended dosage is 0.2 mg once daily with or without food. Known or suspected GI obstruction, increased risk of recurrent obstruction, and hypersensitivity to the medication are contraindications to naldemedine.14 Although renal adjustment is not necessary, patients with severe hepatic impairment should not take this medication. The most common side effects are abdominal pain, diarrhea, nausea, and gastroenteritis. Concomitant use with strong CYP3A4 inducers such as rifampin should be avoided. Moderate and strong CYP3A4 inhibitors and P-glycoprotein inhibitors may increase naldemedine concentrations; therefore, monitoring for adverse reactions is recommended in patients taking these medications.14
Naldemedine was approved based on promising safety and efficacy data from three clinical trials (two conducted over 12 weeks and one conducted over 52 weeks).20,21 However, how this medication fits into clinical practice for the treatment of OIC remains to be determined, especially since it is a Schedule II controlled substance. See TABLE 2 for a summary of important information regarding prescription medications approved for OIC in noncancer pain.8
PHARMACISTS’ ROLE
With the opioid epidemic and the thoughtful use of opioids making headlines, it is more important than ever that pharmacists take a proactive role in counseling patients who are taking opioid medications. Given that OIC is the main side effect of opioids and the cause of reduced quality of life in opioid users, the appropriate prevention, identification, and treatment of OIC are critical areas for pharmacist involvement.6
CONCLUSION
OIC is a common side effect of long-term opioid use. This condition may affect the patient’s activities of daily living. To achieve optimal results, healthcare providers should be familiar with the many different nonpharmacologic and pharmacologic therapies that are available for OIC management.
REFERENCES
1. CDC. U.S. prescribing rate maps. www.cdc.gov/drugoverdose/maps/rxrate-maps.html. Accessed September 11, 2017.
2. Nelson AD, Camilleri M. Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis. 2016;7:121-134.
3. Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Ther Adv Gastroenterol. 2015;8:206-220.
4. Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737.
5. Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269-281.
6. Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137-144.
7. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5.
8. UpToDate online drug database. Waltham, MA: UpToDate; 2013. http://uptodate.com. Accessed September 2017.
9. Amitiza (lubiprostone) package insert. Rockville, MD: Sucampo Pharma Americas, LLC, and Deerfield, IL: Takeda Pharmaceuticals America, Inc; August 2017.
10. Wilson N, Schey R. Lubiprostone in constipation: clinical evidence and place in therapy. Ther Adv Chronic Dis. 2015;6:40-50.
11. Brenner DM, Chey WD. An evidence-based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl. 2014;2:38-46.
12. Relistor (methylnaltrexone bromide) package insert. Bridgewater, NJ: Valeant Pharmaceuticals; January 2017.
13. Movantik (naloxegol) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; August 2017.
14. Symproic (naldemedine) package insert. Florham Park, NJ: Shionogi Inc; March 2017.
15. Rauck R, Slatkin NE, Stambler N, et al. Randomized, double-blind trial of oral methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Pract. 2017;17:820-828.
16. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
17. Webster L, Chey WD, Tack J, et al. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther. 2014;40:771-779.
18. Webster L, Dhar S, Eldon M, et al. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patient with opioid-induced constipation. Pain. 2013;154:1542-1550.
19. Yoon SC, Bruner HC. Naloxegol in opioid-induced constipation: a new paradigm in the treatment of a common problem. Patient Prefer Adherence. 2017;11:1265-1271.
20. Hale M, Wild J, Reddy J, et al. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol. 2017;2:555-564.
21. Webster L, Nalamachu S, Yamada T, et al. Long-term safety and efficacy of naldemedine for the treatment of opioid-induced constipation in subjects with chronic non-cancer pain receiving opioid therapy. Results from a 52-week phase 3 clinical trial. Postgrad Med. 2016;128(suppl 2):5.
To comment on this article, contact rdavidson@uspharmacist.com.






