US Pharm. 2018;43(1):HS2-HS7.
ABSTRACT: Continuous positive airway pressure (CPAP) is the treatment of choice for moderate-to-severe obstructive sleep apnea (OSA). Approximately one-half of all patients with OSA fail to comply with CPAP therapy because of mask-related problems, treatment-related side effects, patient attitude, or perceived lack of benefit. Some patients who are intolerant to CPAP therapy may have anatomical problems that are amenable to surgery. For certain patients with OSA, surgery may be an alternative or adjunct to CPAP therapy. It is essential that pharmacists in all practice settings recognize the morbidity and mortality associated with OSA and be familiar with surgical treatment options in appropriate candidates.
Obstructive sleep apnea (OSA), a common chronic disorder, is caused by a blockage of the upper airway during rapid eye movement (REM) sleep that results in snoring, oxygen desaturations, disrupted sleep, and significant morbidity and mortality.1-3 Upper-airway obstruction occurs when the soft palate and/or tongue collapses posteriorly against the pharyngeal wall because of the loss of normal muscle tone during REM sleep. Epidemiologic studies estimate that 2% to 4% of the adult population has OSA, with selected populations—males, increased BMI (30 or higher), age 60 years and older—more commonly affected.1,3,4 These populations are believed to be at increased risk for airway obstruction because they have more anatomical crowding of the upper airway at baseline.
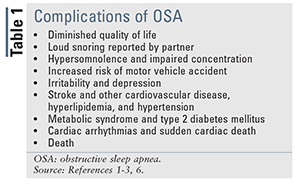
The gold standard for OSA treatment is continuous positive airway pressure (CPAP) therapy (steady flow of air pumped through the nose and/or mouth into the airways of the lungs), which acts as a pneumatic splint to keep the airways patent during sleep. However, owing to mask discomfort, skin irritation, noise, or claustrophobia, up to 60% of patients will not achieve long-term compliance.4,5 Untreated OSA and noncompliance with CPAP therapy must be taken seriously, as these factors increase the risk of multiple health complications (TABLE 1). This article will discuss surgical alternatives to CPAP therapy in adults, their relative effectiveness, and the pharmacist’s role in counseling adult patients with OSA who are considering surgery.
Diagnosis
The American College of Physicians recommends screening for OSA and obtaining a polysomnogram (PSG) in patients with unexplained or excessive daytime sleepiness. The PSG simultaneously records a patient’s brain waves, oxygen saturations, respiratory effort, heart and breathing rates, and eye and leg movements during sleep.3 OSA is confirmed if the PSG shows cyclical airway obstruction with associated oxygen desaturations and sleep arousals. OSA disease severity may be assessed with the apnea-hypopnea index (AHI) and/or respiratory disturbance index (RDI) from the PSG in conjunction with the Epworth Sleepiness Scale (ESS). Depending on insurance status and availability, a modified PSG may be performed in the patient’s home. Home sleep studies are as accurate as a PSG done in a sleep laboratory for diagnosing OSA (but not other sleep disorders), but the cost is much lower.
Nonsurgical Treatment
Effective medical treatments for OSA include weight loss, CPAP, and oral appliances. Because obesity is a risk factor for OSA, weight loss must be a component of OSA treatment in patients with a BMI greater than 25. Effective weight loss leads to reduced levels of CPAP required for airway patency, which improves compliance and, in some patients, may potentially cure OSA. As previously noted, a significant number of patients eventually abandon CPAP therapy. Obstacles to CPAP compliance include mask-related problems (air leaks, skin abrasion, mask discomfort), treatment-related side effects (nasal congestion, dry throat, frequent awakenings, complaints from bed partner), and the patient’s attitude, knowledge level, and perceived benefits and risks.6
Oral appliances such as mandibular-repositioning appliances and tongue-retaining devices—which are worn during sleep—advance the mandible and/or tongue, thereby enlarging the upper airway and reducing airway collapsibility. Because these devices reduce the AHI and RDI more than placebo but less than CPAP, they are considered second-line therapy.1,7 Other devices (nasal strips and valves) have been studied less rigorously and appear to offer little benefit for OSA patients.
Regarding pharmacologic therapy, modafinil is recommended to treat excessive daytime-sleepiness symptoms that occur despite compliance with effective CPAP treatment. Topical nasal corticosteroids (e.g., fluticasone) are useful adjuncts to facilitating CPAP compliance in patients with allergic rhinitis.6,8,9 Multiple therapies—selective serotonin reuptake inhibitors, protriptyline, theophylline, anticholinergics, and estrogen—have been studied, but they lack efficacy and are not recommended for OSA. Supplemental oxygen is not recommended as a primary treatment for OSA. Although cyclical oxygen desaturations occur as a consequence of OSA, supplemental oxygen does not treat airway obstruction, nor does it generally correct hypoxia episodes.
Clinical pharmacists can optimize the well-being of OSA patients by counseling on sleep hygiene, including avoidance of alcohol and other sedatives; discouraging the use of the noneffective therapies listed above; and encouraging CPAP compliance.1,5
Surgical Options
If the patient cannot tolerate CPAP therapy or remains symptomatic despite compliance, surgical airway correction should be considered. Surgical interventions for OSA attempt to alleviate symptoms by improving the strength of the upper airway and lessening the severity of airway obstruction. Although most surgical procedures for OSA are associated with improved clinical outcomes (in symptoms, cardiovascular risk, motor vehicle accidents, quality of life, and mortality), they are generally not considered curative.1 Surgery is a first-line option to treat OSA in children, but is considered a last resort for adults.
Surgical candidates include children with OSA, adults intolerant to CPAP or an oral appliance, adults with anatomical narrowing of the pharynx (e.g., tonsillar hypertrophy, macroglossia, retrognathia), adults with anatomical features that impair proper CPAP mask fit, adults who refuse to wear a CPAP device, and those with no contraindications to anesthesia or surgery. Surgery is generally reserved for patients with severe OSA because the risk-benefit ratio increases with the severity of the underlying disease.10
As previously stated, obesity is a significant risk factor for OSA and must be addressed in the treatment of these patients. Bariatric surgery may be an adjunctive treatment in morbidly obese patients; however, surgical weight loss typically improves, but does not cure, these patients’ OSA. OSA can recur or worsen with postoperative weight gain and typically occurs years after surgery.10
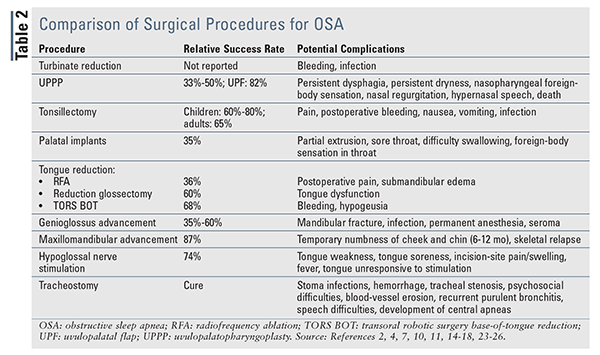
Patients considering surgery must be given information regarding surgical success rates and potential complications of the procedure.10 The procedure(s) chosen must be individualized based on the patient’s unique anatomy and the root cause of upper-airway collapse. Surgical options may focus on correcting the anatomy of the nasal cavity, nasopharynx, oropharynx, and/or hypopharynx, as well as completely bypassing the normal airway. A summary of surgical procedures, their relative success rates, and their potential complications appears in TABLE 2.
Nasal Procedures
Nasal procedures are performed to reduce nasal blockages caused by bone, cartilage, or hypertrophied tissues.4,7 These procedures cannot cure OSA because the origin of apnea is complete obstruction of the pharynx; rather, they are adjuncts that improve breathing and make CPAP more tolerable. When combined with CPAP, nasal procedures allow for reduced CPAP strength, increased CPAP compliance, and improved ESS scores.11,12 The most common nasal surgery is turbinate reduction, which, by debulking hypertrophied turbinates (bones protruding into nasal breathing passage), lessens airway resistance while maintaining normal turbinate functions.13 Radiofrequency turbinate reduction is a minimally invasive strategy for debulking that may be performed under local anesthesia in a physician’s office.
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is the most common surgical procedure for OSA. This method removes excess tissue from the soft palate and pharynx, which are common sites of obstruction in many patients. This surgery requires an overnight stay, and the recovery time may be prolonged.4
Although UPPP is frequently used in patients with mild-to-moderate OSA, its success is relatively low (33%-50%) and its efficacy decreases over time.2,4,14 One study found that, post procedure, the AHI was reduced by only 33% and the RDI remained relatively high at around 30 awakenings per hour.14 Other studies found that although 90% of UPPP-treated patients had improvement in snoring, patient-reported outcomes were much less impressive, with 27% of patients perceiving a good response, 34% perceiving a poor response, and 30% reporting worsened symptoms following surgery.2,7
Other forms of UPPP have shown variable response rates. Uvulopalatal flap (UPF), in which mucosal and submucosal adipose tissue is removed from the tonsils and soft palate, has resulted in an 82% success rate in limited studies.15 UPF has shown favorable responses, but laser-assisted uvulopalatoplasty should be avoided based on reports of postoperative edema and swelling of the upper airway requiring urgent tracheostomy.4,10,14
Tonsillectomy
Tonsillectomy and adenoidectomy are the next most frequently performed procedures. They are most commonly employed in children and should be considered first-line options in pediatric populations.4 These procedures are held to be safe and effective in children older than 2 years, with cure rates around 60% to 80%.4 Tonsillectomy may also be performed in adults with tonsillar hypertrophy. A meta-analysis that examined tonsillectomies in adult patients with tonsillar hypertrophy showed a 65% reduction in the AHI, as well as improvement in ESS scores.16
Palatal Implants
Soft-palate implant surgery, also known as the Pillar procedure, is a minimally invasive strategy for treating snoring and mild cases of OSA.10 Three polyester rods are placed in the soft palate, where they initiate an inflammatory response in the surrounding soft tissues. The resulting fibrosis leads to stiffening of the soft palate and reduced contact between the palate and the back of the pharynx during sleep (i.e., less airway obstruction). This procedure may be performed under local anesthesia. One meta-analysis showed a 35% reduction in AHI and improved ESS scores.14
Hypopharyngeal Procedures
In OSA, the tongue may cause obstruction if it collapses backward excessively during sleep or if the base of the tongue is exceptionally large. This contributes to blockage, particularly in patients who sleep on their back. Available procedures can reduce the size of the tongue or advance it forward out the airway.4
Tongue-reduction procedures include radiofrequency ablation (RFA), reduction glossectomy, and transoral robotic surgery base-of-tongue reduction (TORS BOT). These procedures are performed in patients with mild-to-moderate OSA who cannot tolerate or are unwilling to adhere to CPAP therapy.10 RFA uses electrocautery to help debulk the tongue. It is considered minimally invasive, but multiple treatments over several weeks are required.4,7 RFA cures obstruction in only 36% of patients, but its greatest benefit is in reducing snoring.4,7 Reduction glossectomy, which involves surgical debulking of the tongue, improves AHI and ESS scores significantly, with a surgical success rate of 60%.17 Following surgery, however, taste and sensation may be affected.2 Finally, the relatively new TORS BOT helps decrease the size of the base of the tongue using operative debulking assisted by a surgical robotic device. TORS BOT has shown improvement in AHI and ESS scores and has a reported surgical success rate of 68%.18
Genioglossus advancement, the most commonly performed tongue-advancement procedure, involves moving the genioglossus muscle forward to stabilize the tongue base anteriorly, thereby enlarging the retrolingual airway.4 This procedure is similar to the jaw-thrust maneuver used when intubating or performing cardiopulmonary resuscitation.4 The procedure is considered minimally invasive and may be performed on an outpatient basis. Improvements in daytime sleepiness and quality of life have been reported, and success rates range from 35% to 60%.2,4 Genioglossus advancement is not intended for morbidly obese patients or for those with abnormal mandible development or severe OSA (AHI >30), as high failure rates have been reported in these populations.2,7
Maxillomandibular Advancement
Maxillomandibular advancement (MMA) uses simultaneous advancement of the maxilla and mandible to enlarge the retrolingual airway.4,10 It is indicated for treatment of severe or refractory OSA in patients who cannot tolerate or are unwilling to adhere to CPAP, have failed previous surgical treatments, or have significant maxillomandibular deficiency.4,10 Although MMA has one of the highest efficacy rates (87%) of all surgical options, it should be considered only in extreme cases because it can cause dramatic changes in physical appearance.7 Studies have shown that MMA has sustained clinical success, with 90% of patients maintaining therapeutic benefit at 51 months.19 One study of patient-oriented outcomes showed that 93.3% of patients also had improvements in quality of life, including productivity, social outcomes, and physical-activity level.20
Hypoglossal Nerve Stimulation
The newest surgical treatment for OSA is hypoglossal nerve stimulation, in which an impulse generator is implanted in the chest wall. The generator has an electrical lead that senses diaphragmatic contraction and simultaneously sends an impulse down a second lead that activates the genioglossus muscle through the hypoglossal nerve. This electrical stimulation causes contraction of pharyngeal structures during the inspiratory effort, thus preventing airway collapse and maintaining ventilation.21,22 Early results are promising, including improvements in AHI, oxygen saturations, and ESS scores at 3 and 6 months.21 Prolonged follow-up showed sustained success in 74% of patients; AHI scores remained significantly reduced at 12 and 36 months.22,23 Surgery and device-related complications affect 2% of patients following surgery.22
Tracheostomy
Considered the option of last resort, tracheostomy is the only truly curative treatment for OSA because it completely bypasses the upper airway, including any anatomical or physiological obstructions.4 This surgery is invasive and disfiguring, and it may have a psychosocial impact on the patient’s life. However, it significantly decreases the AHI while consistently improving ESS scores, leading to reduced mortality compared with untreated OSA cohorts.24 Permanent tracheostomy is an option for morbidly obese patients with concurrent obesity hypoventilation syndrome, patients with significant craniofacial anomalies, and OSA patients who have failed other nonsurgical and surgical treatments.
Role of the Pharmacist
Pharmacists, as one of the most accessible healthcare professionals, play an important role in patient education, drug management, and disease-state management. Pharmacists can increase the awareness of OSA, suggest physician screening to patients with characteristic comorbidities (TABLE 1), and promote adherence to CPAP therapy. Pharmacist counseling for OSA patients includes promoting smoking cessation, weight loss, exercise, proper nutrition, sleep hygiene, and medication or CPAP therapy compliance. Although not directly involved in the decision to pursue surgical therapies for OSA, the pharmacist can have a significant effect on outcomes by helping patients understand their options beyond conventional CPAP therapy.
Conclusion
When OSA treatments are being considered, a stepwise approach should be used. Such an algorithm progresses from CPAP alone to CPAP with oral devices and/or medications and, ultimately, to surgical interventions. Generally, surgical correction of structures in the nose, pharynx, tonsils, and tongue should be attempted before more invasive procedures, such as MMA and hypoglossal nerve stimulation. Tracheostomy should be a last-line option in severe disease or in treatment-refractory patients. Following surgery, pharmacists play an important role in assessing complications and therapy benefits and in counseling on the continued use of CPAP based on low cure rates for the surgical procedures. Postoperative sleep studies should be performed judiciously to assess for recurrent disease and to reevaluate the appropriateness of CPAP therapy settings.4
REFERENCES
1. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
2. Mehra P, Wolford LM. Surgical management of obstructive sleep apnea. Proc (Bayl Univ Med Cent). 2000;13:338-342.
3. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
4. Won CH, Li KK, Guilleminault C. Surgical treatment of obstructive sleep apnea: upper airway and maxillomandibular surgery. Proc Am Thorac Soc. 2008;5:193-199.
5. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
6. Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031-1035.
7. Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics. 2012;9:710-716.
8. Wickwire EM, Lettieri CJ, Cairns AA, Collop NA. Maximizing positive airway pressure adherence in adults: a common-sense approach. Chest. 2013;144:680-693.
9. Au TH, Castillo SL, Morrow LE, Malesker MA. Obstructive sleep apnea and continuous positive airway pressure: a primer for pharmacists. US Pharm. 2015;40(1):30-33.
10. Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33:1408-1413.
11. Ishii L, Roxbury C, Godoy A, et al. Does nasal surgery improve OSA in patients with nasal obstruction and OSA? A meta-analysis. Otolaryngol Head Neck Surg. 2015;153:326-333.
12. Camacho M, Riaz M, Capasso R, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. Sleep. 2015;38:279-286.
13. Grymer LF, Illum P, Hilberg O. Septoplasty and compensatory inferior turbinate hypertrophy: a randomized study evaluated by acoustic rhinometry. J Laryngol Otol. 1993;107:413-417.
14. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
15. Li HY, Li KK, Chen NH, Wang PC. Modified uvulopalatopharyngoplasty: the extended uvulopalatal flap. Am J Otolaryngol. 2003;24:311-316.
16. Camacho M, Li D, Kawai M, et al. Tonsillectomy for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2016;126:2176-2186.
17. Murphey AW, Kandl JA, Nguyen SA, et al. The effect of glossectomy for obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;153:334-342.
18. Miller SC, Nguyen SA, Gillespie MB, et al. Transoral robotic base of tongue reduction for obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2017;127:258-265.
19. Riley RW, Powell NB, Li KK, et al. Surgery and obstructive sleep apnea: long-term clinical outcomes. Otolaryngol Head Neck Surg. 2000;122:415-421.
20. Lye KW, Waite PD, Meara D, Wang D. Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2008;66:968-972.
21. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125:1254-1264.
22. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139-149.
23. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154:181-188.
24. Camacho M, Certal V, Brietzke SE, et al. Tracheostomy as treatment for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2014;124:803-811.
25. Levring-Jäghagen E, Nilsson ME, Isberg A. Persisting dysphagia after uvulopalatoplasty performed with steel scalpel. Laryngoscope. 1999;109:86-90.
26. Woodson BT, Robinson S, Lim HJ. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty. Orolaryngol Head Neck Surg. 2005;133:211-217.
To comment on this article, contact rdavidson@uspharmacist.com.






