ABSTRACT: Acute mountain sickness (AMS) is a common disorder, affecting patients who travel to areas of high altitude. Decreased barometric pressure and environmental temperatures associated with higher elevations can cause hypoventilation and hypoxemia. Major risk factors for AMS include rate of ascent, sleeping at a higher elevation, and individual susceptibility. Patients present with headache and additional symptoms such as nausea, vomiting, weakness, and/or anorexia. Nonpharmacologic treatment includes descent, rest, and oxygen therapy. Pharmacologic therapy with acetazolamide and/or dexamethasone is used for treatment and prophylaxis. Other prevention strategies include gradual ascent, sleeping at lower altitudes, and avoidance of alcohol and other depressants.
US Pharm. 2017;42(7)HS-22-HS26.
Each year, large numbers of people travel to places of high altitude for recreational pursuits (e.g., skiing, trekking, mountain climbing) as well as for work and military service. Furthermore, ski-resort towns in the western United States alone attract more than 30 million visitors annually.1,2 As more people travel to higher elevations, the incidence of high-altitude illness (HAI) is also rising. HAI is a term used to describe three distinct syndromes including acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). AMS is the most common, with the reported incidence varying from 25% to 85% worldwide depending on elevation and rate of ascent. Both HACE and HAPE are considered life- threatening, but the incidence is extremely low (0.1% and 4%, respectively). Although AMS is often self-limiting, it can be debilitating, and if untreated, can progress to HACE or HAPE.3,4 Awareness of AMS among pharmacists has become increasingly important. While pharmacists at higher elevations may see patients suffering from AMS and need to be familiar with appropriate treatment, pharmacists at lower altitudes must also be knowledgeable regarding AMS because patients may seek their advice prior to traveling.
Pathophysiology
The pathophysiology of AMS is unclear. However, current evidence suggests that decreased barometric pressure at higher altitudes results in hypoventilation, impaired gas exchange, and hypoxemia. This triggers a neurohormonal and hemodynamic response resulting in fluid retention, which can lead to cerebral edema, increased intracranial pressure, and the development of symptoms seen in AMS.1,3 Additionally, environmental temperatures drop an average of 6.5°C per 1,000-foot gain in elevation, which increases the amount of oxygen required to maintain body temperature.2 The decreased oxygen supply and increased demand at higher altitudes require the body to make adjustments in order to minimize hypoxemia and maintain cellular function. This process is called acclimatization and varies markedly among individuals.5
Epidemiology
Major risk factors associated with the development of AMS include rate of ascent, final elevation reached, sleeping elevation, and individual susceptibility. AMS affects both genders and all ages, although the incidence may be slightly lower in males and anyone aged 50 years or more. Other risk factors include vigorous exercise prior to acclimatization, a history of AMS, and permanent residence at elevations lower than 900 m.1,3,6,7 Substances or conditions that alter ventilation may also increase the risk of AMS. This includes respiratory depressants like alcohol, sedative/hypnotic medications, and comorbid conditions such as moderate-to-severe chronic obstructive pulmonary disease and pulmonary hypertension.5,8,9
Clinical Presentation and Diagnosis
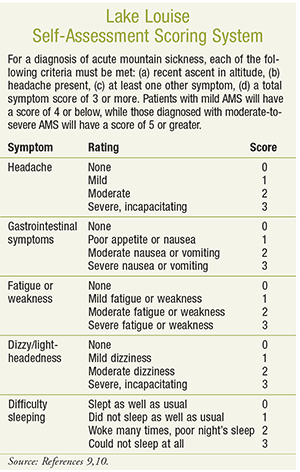
The diagnosis of AMS is based on symptoms, setting, history, and the exclusion of other conditions.8 In 1991, a committee of experts at the International Hypoxia Symposium in Lake Louise, Canada, proposed a quantitative scoring system that evaluates the severity of presenting symptoms (see Lake Louise Self-Assessment Scoring System online at www.uspharmacist.com). Based on this system, AMS may be diagnosed when headache is present together with at least one other symptom (e.g., nausea, weakness, difficulty sleeping) in the setting of a recent gain in altitude.9,10 The nonspecific symptoms seen in AMS are often misdiagnosed as a viral illness, dehydration, alcohol hangover, or exhaustion. A key differentiating factor, however, is the recent change in altitude, as symptoms usually occur within 6 to 12 hours of ascent, although they can appear as early as 1 hour following ascent.1,3,5,9
Nonpharmacologic Treatment
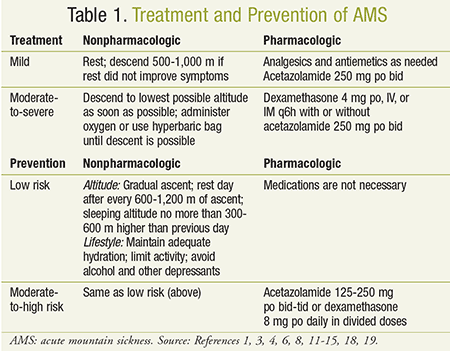
Pharmacists should be able to recognize the manifestations of AMS in order to determine when nonpharmacologic treatment should be recommended and when referral is necessary (see Table 1). Nonpharmacologic options include stopping, resting, and/or descent.6,11-14 If AMS is mild, stopping, resting, and increasing fluid intake may be sufficient treatment.3,8,12,14-16 Avoiding further ascent, alcohol, and sedatives will expedite symptom resolution, whereas additional ascent or exertion may cause symptom progression.1,12,14,15 If resting does not yield improvement, descent of 500 to 1,000 m is recommended.1,4,6,11 When symptoms are severe or worsen, or there is concern for HACE or HAPE, descent can be lifesaving; supplemental oxygen or treatment with a portable hyperbaric bag should be administered until descent occurs.3,6,8,11,13,14,17 Access to oxygen at higher altitudes may be limited, but, if available, patients should see a response within an hour.8,11 Hyperbaric bags, or chambers, simulate a descent that can equate to several thousand feet.1,8 Their advantages include portability due to their compact size and low weight.1,8 Limitations on use include symptom recurrence once the individual is removed from the bag, and the occurrence of claustrophobia and/or vomiting.11
Pharmacologic Treatment
Pharmacologic options for AMS primarily include acetazolamide (Diamox) and dexamethasone, in addition to medications for symptomatic treatment such as analgesics and antiemetics. Acetazolamide, a carbonic anhydrase inhibitor, can alleviate acute symptoms of mild AMS by accelerating acclimatization.1,8,11,14,18 The recommended adult dosage is 250 mg orally twice daily; in the pediatric population, the dosage is 2.5 mg/kg per dose twice daily up to a maximum of 250 mg per dose.1,5,8,11,13,14,16,18 Acetazolamide should not be used in people with a history of a sulfa allergy, hepatic disease or marked renal impairment, adrenocortical insufficiency, hyponatremia, hypokalemia, or metabolic acidosis.18 Common adverse effects include paresthesias (peritorbital region, hands, and feet), dysgeusia (especially with carbonated beverages), glycemic and/or electrolyte changes, diuresis, and metabolic acidosis.1,4,10,14,18 Elderly patients, patients with diabetes mellitus, and those taking concurrent high-dose aspirin may be more likely to experience these adverse effects.18
Dexamethasone, a long-acting corticosteroid, is an effective treatment for any degree of AMS and should be used in patients with sulfa allergy or preferentially in those with moderate-to-severe AMS.1,8,11,14,19 The dosing for dexamethasone is 4 mg every 6 hours administered orally, IV, or IM until symptoms resolve.8,11,14,16,19,20 For pediatric patients, the dosage is 0.15 mg/kg per dose every 6 hours.1,11,13,19 Although acetazolamide assists with acclimatization, dexamethasone does not, and patients may experience rebound AMS upon discontinuation.1,4,19,20 Dexamethasone is contraindicated in patients with systemic fungal infections. It should be used cautiously in patients with cardiovascular disease, gastrointestinal disease, diabetes mellitus, psychiatric disorders, and hepatic or renal impairment.19 Common adverse effects of dexamethasone include hyperglycemia, changes in mood, fluid retention, and dyspepsia.14,19 Antacids may decrease the bioavailability of dexamethasone, while strong CYP3A4 inducers may decrease the serum concentrations.19 Acetazolamide and dexamethasone may be used alone or in combination.12 If symptoms progress rapidly or descent will be delayed, then combination therapy is preferred.8
For symptomatic treatment, OTC analgesics (e.g., aspirin, ibuprofen, naproxen, acetaminophen) at usual dosages successfully treat AMS-associated headache.1,8,14,16 However, opioid analgesics should be avoided in these patients because of their respiratory adverse effects. Nausea and vomiting may be treated with antiemetics such as promethazine or ondansetron. Insomnia may be treated with acetazolamide, which improves nocturnal oxygenation.1,8
Prevention
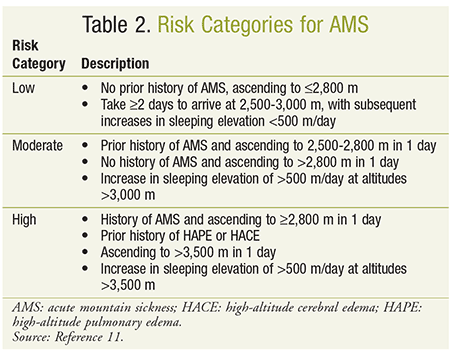
Pharmacists may be faced with questions regarding strategies to prevent AMS. Current guidelines provide recommendations based on patient risk factors including a prior history of AMS, rate of ascent, and maximum altitudes for climbing and sleeping (see Table 2). Low-risk patients should rely solely on nonpharmacologic strategies to prevent AMS, whereas patients with a moderate-to-severe risk may benefit from both behavioral approaches and pharmacologic prophylaxis (see Table 1).11
Gradual ascent is considered the most effective approach to prevent the development of AMS.8 Prior to ascending to higher elevations, it is recommended that patients sleep at an altitude of 1,500 to 2,500 m to help with acclimatization.3,4,14 Those traveling above 2,500 m should ascend at no more than 300 to 600 m/day above the sleeping altitude of the previous night.1,3,11 Adding a rest day after every 600 to 1,200 m of ascent will also assist the acclimatization process.1 When gradual ascent is not possible, individuals may ascend faster than the recommended rate if they return to lower elevations for sleep (climb high, sleep low). Other nonpharmacologic strategies include maintaining adequate hydration, limiting activity for the first few days at higher elevations, and avoiding alcohol and other depressants.8,14
In moderate-to-severe risk situations, acetazolamide is the preferred agent, with recommended dosing of 125 to 250 mg orally two to three times daily, preferably initiated 24 to 48 hours before ascent and continued for the first day or two at high altitude.1,4,8,11,14,21 Although drug prophylaxis should be avoided in children, there are cases when it may be necessary. The pediatric dosage of acetazolamide is 2.5 mg/kg per dose twice daily (125 mg/dose maximum).11,13 For patients who are intolerant or allergic to acetazolamide, dexamethasone 8 mg daily in divided doses may be considered.3,4,8,9,11 Duration should be limited in order to prevent glucocorticoid toxicity and/or adrenal suppression. Dexamethasone should not be used for prevention in the pediatric population.11 Although the combination of acetazolamide and dexamethasone was found to be more effective than either given alone, use of the combination should be reserved for when more severe symptoms of AMS are likely.22
Several studies have focused on the utility of analgesics in AMS. Prophylaxis with aspirin has shown a significant reduction in the development of AMS-associated headache at a dosage of 320 mg orally taken at 4-hour intervals, starting 1 to 2 hours prior to arrival at final altitude, for a total of three doses.23,24 In addition, ibuprofen has reduced the incidence of AMS and AMS-associated headache at a dosage of 600 mg three times daily, starting 6 to 24 hours before ascent and continuing for a minimum of three doses.25,26
Ginkgo biloba is an herbal product that may be effective for prophylaxis of AMS. Oral dosages used in clinical trials range from 60 mg three times daily to 80 mg to 120 mg twice per day started prior to ascent.14,27-29 Although there is conflicting evidence regarding ginkgo's effectiveness, the lack of a standardized preparation may partially account for the variation in results.11,29 Current guidelines do not recommend this agent for prophylaxis; however, patients may choose to proactively initiate the medication because it is readily available OTC.
Antioxidant vitamin supplements may also be helpful in preventing AMS; however, supporting data were found only in a single clinical trial. Patients were randomized to receive antioxidant supplements (each containing ascorbic acid, tocopherol, and lipoic acid) or placebo 3 weeks prior to travel and continued while at high altitude. Although there was no significant difference in the incidence of AMS, participants given antioxidant prophylaxis demonstrated fewer AMS-related symptoms than those who were not given prophylaxis.30 Additional research with larger numbers of patients is needed before this intervention can be routinely recommended.14
Conclusion
Millions of people each year travel to areas of higher altitude. Because pharmacists are easily accessible healthcare professionals, they are uniquely positioned to assist patients who are already at their destination or are preparing to travel. Therefore, it is essential that all pharmacists are able to educate their patients on treatment options for AMS and the best available prevention strategies.
REFERENCES
1. Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345(2):107-114.
2. Hackett PH, Roach RC. High altitude medicine. In: Auerbach PS, ed. Wilderness Medicine. 5th ed. Philadelphia, PA: Mosby Elsevier; 2007.
3. Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967-1974.
4. Fiore DC, Hall S, Shoja PS. Altitude illness: risk factors, prevention, presentation and treatment. Am Fam Physician. 2010;82(9):1103-1110.
5. Harris MD, Terrio J, Miser WF, et al. High-altitude medicine. Am Fam Physician. 1998;57(8):1907-1914.
6. Bartsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med. 2013;368:2294-2302.
7. Schneider M, Bernasch D, Weymann J, et al. Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med Sci Sport Exer. 2002;34:1886-1891.
8. Ellsworth A. Pharmacotherapy for altitude-related illness. J Pharm Pract. 2003;16(1):68-75.
9. Imray C, Booth A, Wright A, et al. Acute altitude illnesses. BMJ. 2011;343:411-417.
10. Plant T, Aref-Adib G. Travelling to new heights: practical high altitude medicine. Brit J Hosp Med. 2008;69(6):348-352.
11. Luks AM, McIntosh SE, Grissom CK, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014;25:S4-S14.
12. Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin N Am. 2004;22:329-355.
13. Pollard AJ, Niermeyer S, Barry P, et al. Children at high altitude: an international consensus statement by an ad hoc committee of the International Society for Mountain Medicine. High Alt Med Biol. 2001;2(3):389-403.
14. Spooner LM, Olin JL, DeBellis RJ. Pharmacotherapy of high-altitude illness. Am J Lifestyle Med. 2007;1(2):129-141.
15. Duster MC, Derlet MN. High-altitude illness in children. Pediatr Ann. 2009;38;(4):218-223.
16. West JB. High-altitude medicine. Am J Respir Crit Care Med. 2012;186(12):1229-1237.
17. Kasic JF, Yaron M, Nicholas RA, et al. Treatment of acute mountain sickness: hyperbaric versus oxygen therapy. Ann Emerg Med. 1991;20(10):1109-1112.
18. Acetazolamide. Lexi-Comp Online. Hudson, OH: Lexi-Comp, Inc; 2017. https://online.lexi.com. Accessed June 1, 2017.
19. Dexamethasone. Lexi-Comp Online. Hudson, OH: Lexi-Comp, Inc; 2017. https://online.lexi.com. Accessed June 1, 2017.
20. Hackett PH, Roach RC, Wood RA, et al. Dexamethasone for prevention and treatment of acute mountain sickness. Aviat Space Environ Med. 1988;59(10):950-954.
21. Basnyat B, Gertsch JH, Holck PS, et al. Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy trial. High Alt Med Biol. 2006;7(1):17-27.
22. Bernhard WN, Schalick LM, Delaney PA, et al. Acetazolamide plus low-dose dexamethasone is better than acetazolamide alone to ameliorate symptoms of acute mountain sickness. Aviat Space Environ Med. 1998;69(9):883-886.
23. Burtscher M, Likar R, Nachbauer W, et al. Aspirin for prophylaxis against headache at high altitudes: randomized, double blind, placebo controlled trial. BMJ. 1998;316:1057-1058.
24. Burtscher M, Likar R, Nachbauer W, et al. Effects of aspirin during exercise on the incidence of high-altitude headache: a randomized, double-blind placebo-controlled trial. Headache. 2001;41:542-545.
25. Gertsch JH, Corbett B, Holck PS et al. Altitude sickness in climbers and efficacy of NSAIDs trial (ASCENT): randomized, controlled trial of ibuprofen versus placebo for prevention of altitude illness. Wilderness Environ Med. 2012;23:307-315.
26. Lipman GS, Kanaan NC, Holck PS, et al. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59:484-490.
27. Gertsch JH, Basnyat B, Johnson EW, et al. Randomised, controlled trial of ginkgo biloba and acetazolamide for prevention of acute mountain sickness: the prevention of high altitude illness trial. BMJ. 2004;328:797-799.
28. Chow T, Browne V, Heileson HL, et al. Ginkgo biloba and acetazolamide prophylaxis for acute mountain sickness. Arch Intern Med. 2005;165:296-301.
29. Leadbetter G, Keyes LE, Maaekstad KM, et al. Ginkgo biloba does and does not prevent acute mountain sickness. Wilderness Environ Med. 2009;20(1):66-71.
30. Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant supplementation at high altitude. High Alt Med Biol. 2001;2:21-29.
To comment on this article, contact rdavidson@uspharmacist.com.