US Pharm. 2017;42(2):HS22-HS25.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
ABSTRACT: The use of triple therapy in patients after percutaneous coronary intervention is becoming more common because numerous patients requiring cardiac catheterization have risk factors for other disease states that necessitate anticoagulation, namely, atrial fibrillation. Although patients may have appropriate indications for all three medications—which often include aspirin, a thienopyridine, and an anticoagulant—concomitant use of these agents tends to result in higher rates of bleeding. Therefore, as a quality and safety measure at one Veterans Affairs facility, patients on triple therapy were identified and had an intervention to alter the medication regimen to preemptively reduce bleeding rates. Ninety-five percent of patients had an adjustment in their medication regimen without any observed instances of revascularization at follow-up. A review of safety outcomes showed that no patient experienced an admission or an adjustment of medication therapy related to gastrointestinal bleeding.
Triple antithrombotic therapy (TAT) is defined as the concomitant administration of dual antiplatelet therapy (DAPT) with anticoagulation. DAPT commonly involves aspirin in combination with either ticagrelor (a cyclopentyltriazolopyrimidine [CPTP]) or a thienopyridine, such as clopidogrel or prasugrel. Current guidelines recommend that these drug classes be used after percutaneous coronary intervention (PCI) with a bare-metal stent (BMS) or a drug-eluting stent (DES) to reduce the risk of stent thrombosis. Mortality associated with stent thrombosis is estimated at 20% to 45%, with the most immediate risk seen within the first 30 days of stent placement.1 Indications for anticoagulation include atrial fibrillation (AF), venous thromboembolism, and left ventricular mural thrombus, as well as other disorders.2
The CDC reports that, in the United States, an estimated 2.7 to 6.1 million people have AF. Risk factors for the development of AF include hypertension, obesity, increased age, diabetes mellitus, structural and/or ischemic heart disease, hyperthyroidism, heavy alcohol use, and heart failure.3 In patients with diagnosed nonvalvular AF, the 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society atrial fibrillation guideline recommends that patients with diagnosed nonvalvular AF who are deemed to be at high risk for stroke should be maintained on anticoagulation with either a vitamin K antagonist or a newer direct oral anticoagulant.4 Risk is assessed according to the CHA2DS2-VASc score, which is a scoring metric used to stratify the estimated annual ischemic stroke risk in patients with multiple comorbidities and independent risk factors; a score of ≥2 warrants anticoagulation.4 Similarly, risk factors for the development of coronary heart disease tend to overlap with those associated with the occurrence of AF, including hypertension, diabetes mellitus, and obesity, which may lead to PCI and more frequent use of TAT in this patient population with comorbid conditions.5
However, there is concern over the greater bleeding risk with TAT, which is associated with increased mortality in post-PCI patients.6 In a meta-analysis conducted by Gao and colleagues, triple therapy was observed to decrease the risk of ischemic stroke, but it was associated with an increase in major bleeding.7 In a study published in 2010, Hansen and colleagues reviewed bleeding rates associated with single, dual, and triple therapies. The primary outcome reviewed was bleeding, which was further stratified into fatal and nonfatal bleeds. The combination of aspirin, clopidogrel, and warfarin was associated with more than a threefold increase in both fatal and nonfatal bleeding events compared with warfarin monotherapy.8 Therefore, strong consideration of risk versus clinical benefit in patients requiring TAT became a primary concern, and it was not until 2013, when new clinical evidence surfaced, that an alternative to TAT in this selected patient population was considered. WOEST (What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing) was an open-label, randomized, controlled trial in patients on anticoagulation therapy who were undergoing PCI. Patients were assigned to receive anticoagulation plus clopidogrel or TAT (anticoagulation plus aspirin and clopidogrel). At 1-year follow-up, significantly lower rates of bleeding were seen in the dual-therapy group compared with the triple-therapy group, without an increase in cardiovascular events including any myocardial infarction, any target-vessel revascularization, any stroke, or any stent thrombosis.9
Quality-Improvement Project
Based on clinical evidence, a quality-improvement project undertaken in collaboration with cardiology and pharmacy services was initiated at one veterans’ facility to evaluate patients on TAT with a cardiac indication to preemptively reduce bleeding rates by appropriately altering therapy according to the WOEST trial recommendations.
Methods
Patients were identified from an initial aggregate report of 4,156 patients with active outpatient orders as of quarter 3 (April-June), 2015, for at least one of the following medications: apixaban, aspirin, aspirin-dipyridamole, cilostazol, clopidogrel, dabigatran, pentoxifylline, prasugrel, rivaroxaban, ticagrelor, and warfarin. Patients were defined as being on TAT if they were taking two antiplatelet medications (aspirin, aspirin-dipyridamole, cilostazol, clopidogrel, pentoxifylline, prasugrel, ticagrelor) in combination with an anticoagulant (apixaban, dabigatran, rivaroxaban, warfarin). A thorough retrospective chart review of 27 patients identified from the aggregate report as being on TAT at the Dayton Veterans Affairs Medical Center (DVAMC) was performed. This review was conducted in conjunction with the cardiology department to specifically address patients on TAT after PCI.
Of the 27 patients reviewed, seven (five vascular patients, one orthopedic patient, and one neurology patient) were on TAT unrelated to PCI and were excluded from intervention. Therefore, 20 patients receiving TAT at the DVAMC underwent intervention. All 20 patients were reviewed and discussed with a DVAMC interventional cardiologist before any recommendation was made to providers.
Patient data were obtained from the Computerized Patient Record System (CPRS). Data recorded included name, sex, Social Security number, medication history (names, doses, start dates, and indications for reviewed medications), dates and types of cardiac procedures (including placement location and type of stent during PCI), and gastrointestinal (GI) interventions related to a possible bleed.
Because this was a quality-improvement project at the DVAMC, Institutional Review Board approval was not required. The local Pharmacy and Therapeutics Committee approved the use of a progress note in the electronic medical record (eMAR) to intervene and recommend medication changes in the interest of patient safety. The internal medicine clinical pharmacy specialist created a note template in CPRS to make a recommendation on therapy modification that was sent to the pharmacist-run anticoagulation clinic, the primary care provider, and the cardiologist for acknowledgment of the recommendation and to stimulate further action. The recommendation consisted of evidence-based results from the WOEST trial suggesting that, in patients on TAT, aspirin could be stopped and the patient could be continued on the additional antiplatelet agent plus anticoagulation. If there was not a lifelong indication for use of either a CPTP or thienopyridine, aspirin therapy should be temporarily suspended and then resumed, in combination with anticoagulation, when the CPTP or thienopyridine was discontinued.
Results
Of the 20 patients reviewed, 19 (95%) were male. Nineteen patients were converted from TAT to dual therapy as a local safety initiative to potentially reduce the known increased bleeding risk seen in the literature. The sole patient not converted to dual therapy received follow-up from a private cardiologist outside of the DVAMC. Based on outpatient cardiology records, the patient was to continue DAPT plus anticoagulation until the fall of 2016, when a reassessment would be made.
Before the progress note with the recommended intervention was placed in the eMAR, eight of the 19 patients (42%) had already been converted from TAT to dual therapy—most often by a DVAMC clinical pharmacy specialist—in both inpatient and outpatient settings. In the remaining 11 patients (58%), the providers accepted the recommendation to discontinue TAT after the intervention was documented in the eMAR by the clinical pharmacy specialist (TABLE 1).
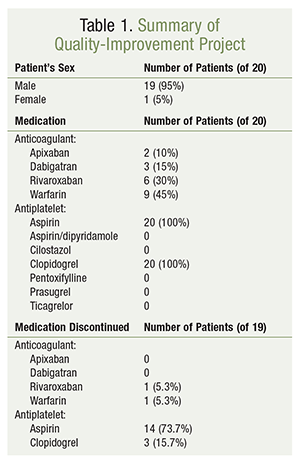
Data collection commenced in August 2015 and was completed in June 2016. A follow-up review of all 20 patients revealed no documentation of admissions related to GI bleeding or repeat coronary interventions in any patient, according to CPRS records during this 10-month time period. Three patients (15%) were admitted to the hospital with indications for either a potential bleeding event or cardiac rule-out: one case of anemia and two cases of cardiac symptoms, such as chest pain or shortness of breath (TABLE 2).
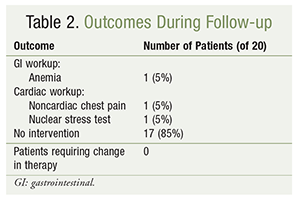
The patient who was admitted for anemia was followed by a gastroenterologist and had an endoscopy in September 2015 and a colonoscopy in May 2016; neither procedure revealed a bleeding source. Of the two patients who presented for cardiac evaluation, one had shortness of breath and underwent a nuclear stress test in March 2016 that revealed no ischemia; the other patient presented with chest pain that, in May 2016, was determined to be noncardiac in nature and required no intervention. Consequently, after evaluation, all three patients were discharged without incident and with no changes in their antiplatelet and anticoagulant medications.
Discussion
In this retrospective study, the use of TAT was identified in a small number of patients within the DVAMC. The majority of patients were receiving TAT for a cardiac indication after PCI. The collaboration between cardiology and pharmacy services allowed for modification of this potentially high-risk bleeding therapy in 95% of the patient population reviewed. When outcomes were assessed after intervention in the 20 TAT patients, no patient had an occurrence of a GI bleed or the need for repeat coronary intervention during follow-up, according to the CPRS medical record.
Strengths of this retrospective review include the consistency of data aggregation, as there was only one pharmacy investigator participating in chart reviews and subsequent documentation of interventions. Similarly, all patient cases were presented to the same interventional cardiologist for assessment and discussion. Although the sample size was small, this study revealed that cardiologists at the DVAMC were cognizant of the risks associated with TAT and tended to prescribe this combination to a limited number of patients to avoid increased rates of bleeding and mortality.
This study also had several limitations; however, the proposed review was undertaken as a quality-assurance project within the institution. One limitation was that this analysis was performed retrospectively. Also, the sample size of identified patients was quite small (N = 27), and only 20 patients had a cardiac indication for TAT. The patient sample was obtained from one medical center, with an overwhelmingly male population consistent with that of veterans. Individual international normalized ratio (INR) goals were not evaluated to determine whether patients taking warfarin as anticoagulation therapy had a preemptively reduced INR goal of 2 to 2.5 to curb bleeding rates, which is sometimes implemented in clinical practice in patients also receiving DAPT. Additionally, patients in this retrospective review were taking only aspirin or clopidogrel, which may have led to a decreased rate of observed GI bleeding compared with the newer antiplatelet agents prasugrel and ticagrelor.
Abundant literature has been published that warns of the increased bleeding risk seen with TAT; however, organizations such as the ACC Foundation and the AHA have provided little guidance for providers on appropriate therapy in post-PCI patients. The WOEST trial displayed promising results in reducing therapy to dual-agent use (clopidogrel plus warfarin) without an increase in cardiovascular events, but it had limitations that may preclude applicability to every TAT patient. Specifically, it was an open-label, non–placebo-controlled study that was not powered to detect a difference in the occurrence of thrombotic events in the dual-treatment arm. Additionally, time in therapeutic range of warfarin was not monitored to determine whether this affected bleeding rates in either treatment group, and there was a higher rate of DES use, rather than BMS use, which led to a longer duration of clopidogrel therapy compared with prior published results of bleeding outcomes in clinical trials.9 Therefore, in the absence of definitive guideline recommendations, providers must continue to assess the need for TAT on a case-by-case basis depending on the individual bleeding risk of the patient.
Education of providers on the increased risk of fatal and nonfatal bleeding with TAT use is the best way to increase awareness of this life-threatening possibility. Performing detailed medication reconciliation at provider appointments and obtaining appropriate medical histories to support indications for antiplatelet and anticoagulant therapies are paramount in preventing inappropriate use or overuse of these high-alert medications. According to the Institute for Safe Medication Practices, high-alert medications have a substantial risk of causing patient harm when used “in error.”10 Therefore, the implementation of multidisciplinary teams, which include clinical pharmacists, is another potential way to ensure appropriate and accurate drug-utilization reviews, including proper durations of therapy.
The creation of a randomized, placebo-controlled trial that follows patients initiated on TAT compared with dual therapy after PCI, with either a CPTP or thienopyridine and an anticoagulant, would be extremely beneficial for future direction. Results of this type of prospective trial could more effectively measure clinical outcomes regarding fatal and nonfatal bleeds and the occurrence of cardiac events, which would help elucidate for practicing clinicians the safest and most effective way to manage this complex patient population.
Conclusion
In this retrospective review of a veteran population on TAT for a cardiac indication, 19 of the 20 patients (95%) underwent an adjustment in the triple-therapy regimen to preemptively reduce the risk of bleeding. Consequently, no episodes of bleeding were found in the population reviewed; neither was the need for revascularization. Further studies are needed to support the findings of this retrospective review in terms of both safety and efficacy with modification of TAT therapy.
REFERENCES
1. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44-e122.
2. Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J. 2004;147:463-467.
3. CDC. Atrial fibrillation fact sheet. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm. Accessed June 1, 2016.
4. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
5. National Heart, Lung, and Blood Institute. What are coronary heart disease risk factors? www.nhlbi.nih.gov/health/health-topics/topics/hd. Accessed June 1, 2016.
6. Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary invervention: results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654-664.
7. Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol. 2011;148:96-101.
8. Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
9. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107-1115.
10. Institute for Safe Medication Practices. ISMP list of high-alert medications in acute care settings. www.ismp.org/Tools/highalertmedications.pdf. Accessed June 9, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





