US Pharm. 2018;43(5):HS8-HS12.
ABSTRACT: Congenital diaphragmatic hernia (CDH) and myelomeningocele (MMC) are congenital birth defects that have lifelong sequelae. Both abnormalities can be treated with supportive care after birth or with fetal surgical interventions. Advances in imaging technology have led to increased precision and better understanding of fetal pathology, but this knowledge has not translated to improvements in prenatal management. Research is ongoing as to whether pre- or postnatal interventions result in better short-term or long-term outcomes. Pharmacists can take an active role in managing pregnant patients or those planning to become pregnant. CDH and MMC are related to inadequate vitamin A and folic acid intakes, respectively, and proper counseling can mitigate these risks. Pharmacists must be aware of recommended dietary allowances and medications that can reduce levels of vitamin A or folic acid.
The last half-century has seen dramatic technological advances that have taken fetal surgery from the pages of science fiction to real-world applications. The first fetal surgery (nonhuman) dates back to 1884, and the first human fetal procedure was performed in 1963.1,2 The introduction of prenatal ultrasound in the 1970s shed light on the nebulous nature of fetal interventions and ushered in new fetal sampling and imaging techniques that helped elucidate fetal pathophysiology.3 Even given a better understanding of the science, congenital birth defects still affect 3% of infants in the United States and can have negative implications for morbidity and mortality.4 The management of patients undergoing fetal surgery requires a multidisciplinary approach that is in the best interest of both the fetus and the mother.
This article will focus on two conditions that may require fetal surgical intervention and on the role pharmacists can play in the management of patients in these circumstances.
Neural Tube Defects
The term neural tube defects (NTDs) denotes a group of disorders that develop during the third or fourth week after conception when a segment of the neural tube does not close properly. The failure of the neural tube to close exposes the spinal cord to the circulating amniotic fluid.5 NTDs may be considered open or closed. Open NTDs (in which the defect is covered by a membrane, or sometimes not covered at all) account for approximately 80% of all NTDs.5 Closed NTDs, which account for the remaining 20%, will not be discussed in this article.
One of the most common open NTDs is myelomeningocele (MMC), also known as spina bifida. In the U.S., MMC has an incidence of 3.4 per 10,000 live births.6 MMC is associated with a 10% mortality rate for live births, and infants who survive are hindered by life-long sequelae that include cognitive issues, hydrocephalus, hindbrain herniation, motor/sensory defects, and fecal and urinary incontinence.7-10 The pathogenesis is explained by the two-hit hypothesis, with the resulting damage thought to be due to a combination of failed neurulation and continuous exposure of the spinal cord to chemicals circulating in the amniotic fluid.11,12 Arnold-Chiari malformation II (A-CII), which occurs in the majority of MMC cases, involves the descent of the brainstem (hindbrain herniation), which then extends into the spinal canal and interferes with the circulation of cerebrospinal fluid (CSF). The hindbrain herniation leads to developmental brain abnormalities and hydrocephalus. To help reroute CSF to the peritoneal cavity, a shunt is placed that will require lifetime monitoring.13 The A-CII abnormality results in cognitive functional impairment, and the adverse effects of hydrocephalus and shunting create a synergy of motor problems.13
For many years, MMC treatment was limited to postnatal interventions.14 The exposed spinal canal was closed after birth, and the patient was subjected to life-long supportive care.15 As imaging technology progressed, more prenatal interventions were performed, and by 2003 more than 200 prenatal MMC repair procedures had been performed. A review of these cases suggested that prenatal interventions were associated not only with an improvement in hindbrain herniation, but also an increase in maternal risk.16,17
To determine the efficacy and safety of prenatal versus postnatal intervention, Adzick and colleagues conducted the Management of Myelomeningocele Study, which took place between 2003 and 2010 and enrolled 183 women.13 The two primary outcomes were 1) fetal/neonatal death or the need for shunt placement at age 12 months and 2) a composite score of the child’s motor function at age 30 months as determined by the Mental Development Index (MDI) of the Bayley Scales of Infant Development II. Secondary outcomes included pregnancy and surgical complications and neonatal morbidity/mortality, and infant secondary outcomes included development of the A-CII formation and motor function according to the MDI.13 Study results favored prenatal surgery, and the trial was terminated early, in 2010. Prenatal surgery was shown to decrease the risk of death or shunt placement at 12 months while also resulting in better motor and mental function at age 30 months.13 Compared with postnatal surgery, prenatal intervention also improved sequelae associated with the A-CII formation, including neuromotor function and independent ambulation.13 Although prenatal intervention was associated with better overall performance, it was also correlated with higher rates of preterm birth, operative complications, and more maternal transfusion during delivery.13
The Pharmacist’s Role in NTD Prevention
Although pharmacists do not assist with actual fetal surgeries, they can do much to help prevent the development of NTD. Over the years, several studies have shown a link between NTD development and inadequate folic acid intake. One such study performed by the British Medical Research Council showed that high-dose folic acid supplementation (4.0 mg/day) in patients who had a previous pregnancy affected by NTDs reduced subsequent NTD development by 70%.18 On the basis of this and other trials, the CDC began recommending that all women of childbearing age take folic acid 0.4 mg daily to prevent MMC and other NTDs. Additionally, the CDC recommends that any woman who has already had an NTD-related pregnancy should consume folic acid 0.4 mg daily regardless of pregnancy status. When these patients are planning a pregnancy, they should increase intake to 4 mg daily 1 month prior to attempting pregnancy and should continue that dosage through the first 12 weeks of pregnancy.19
Along the same lines, pharmacists should be aware of any medications that can cause a reduction in folic acid levels. Many medications can decrease folic acid levels, including certain anticonvulsants (phenytoin, valproic acid, carbamazepine), antibiotics (trimethoprim, pyrimethamine), antihistamines (famotidine, ranitidine), and OTC nonsteroidal anti-inflammatory drugs.20 Pharmacists should take an active role in identifying pregnant patients who are taking these medications and should counsel them about the potential risks to the fetus.
Congenital Diaphragmatic Hernia
Congenital diaphragmatic hernia (CDH) is a severe developmental birth defect of the diaphragm that occurs in approximately one in every 2,500 live births; it represents 8% of all significant birth defects and has a high mortality rate.21,22 The diaphragm, a dome-shaped skeletal muscle that separates the abdominal and thoracic cavities, is divided into two separate muscle groups: the costal diaphragm and the crural diaphragm. CDH occurs when a defect in the structural integrity of the diaphragm allows the visceral contents of the abdomen to herniate into the thoracic cavity, preventing the heart and lungs from maturing to proper dimensions.23
The majority of CDH cases involve defects of the costal diaphragm and are classified into two different types: Morgagni and Bochdalek.23,24 Morgagni hernias result from defects in the anterior portion of the diaphragm, whereas Bochdalek hernias occur in the lumbocostal triangle of the posterolateral wall of the thorax. Bochdalek hernias are also associated with respiratory failure and pulmonary hypoplasia.23 The most severe cases of CDH have multiple organ-system problems involving the lungs, heart, and gastrointestinal tract. As many as 20% of all CDH patients have cardiac structural anomalies, including right-sided heart failure and decreased size of the left-sided heart structures.23,25 Although the physical integrity of the diaphragm is important, the resulting pulmonary hypertension and other developmental sequelae are actually responsible for the high mortality and long-term disability associated with CDH.26,27
For many years, it was believed that the mechanical restriction of the thoracic cavity was the sole reason for the abnormal lung development. More recently, however, a dual-hit hypothesis (similar to that of MMC) has proposed that primary lung defects occur independently of mechanical thoracic compression.23,28,29 A recent investigation by Derderian and colleagues showed that CDH patients have more severe pulmonary, lung, and vascular defects compared with patients whose thoracic cavity is affected by other pulmonary-airway malformations.23,30
Presently, CDH is treated with postnatal surgical repair of the diaphragm and concomitant supportive care. For postnatal patients who are unable to adjust from fetal circulation, extracorporeal membrane oxygenation may be required.23 Enhancements in respiratory therapy management have improved survival rates of CDH postnatally; however, prenatal intervention may be required for patients with low fetal lung volumes because they have the highest mortality rates.23 The goal of fetal intervention for CDH is to reverse the mechanical compression of the thoracic cavity via a procedure known as fetal endoscopic tracheal occlusion (FETO). FETO involves intentionally obstructing the trachea with a balloon in order to cause fluid to accumulate in the lungs, which helps the lungs expand and force the abdominal organs downward. The balloon is placed at 26 to 28 weeks’ gestation, and a second procedure is performed at 34 weeks to remove it.23,31
Despite technological advances in imaging and surgical techniques, prenatal CDH repair remains controversial. A study by the University of California–San Francisco that compared the FETO prenatal intervention with planned delivery and supportive care showed no significant improvement in survival rate, and the study was concluded early without further enrollment when it was determined that 90-day survival rates would be indistinguishable.32 These results have called the efficacy of FETO into question, and the surgical community is awaiting results of an ongoing international randomized trial (Tracheal Occlusion To Accelerate Lung Growth) to determine whether prenatal interventions can reduce mortality and morbidity in CDH.31-35
The Pharmacist’s Role in CDH Management
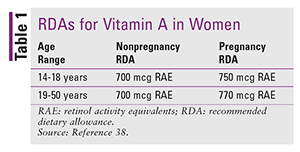
As with NTDs, the pharmacist is not involved in surgical interventions but can still play a valuable role in managing CDH. A 2006 study by Clugston and colleagues found that vitamin A deficiency is associated with the development of Bochdalek type CDH in the rodent model.36 Pharmacists should counsel patients on proper vitamin A intake and know the signs of low vitamin A levels. Pharmacists should also understand the conversion from IU to retinol activity equivalents (RAE). RAE is the unit of measure used by nutrition scientists; however, all food and dietary supplements list vitamin A content in IU. One IU of retinol is equal to 0.3 mcg of RAE. The recommended dietary allowance (RDA) of vitamin A is given in TABLE 1.37,38
The most common sign of low vitamin A levels is xeropthalmia (dry eyes). Other symptoms include light sensitivity, blurred vision, and excessive tearing. A CDH patient experiencing these symptoms should have other diagnoses ruled out because high-dose vitamin A supplementation also may be harmful. High levels of vitamin A have been associated with birth defects, so women who may be pregnant should not take high vitamin A doses.37-39
Conclusion
Great strides have been made in technology and imaging with regard to fetal surgery in the last few decades; however, these advances have not always been associated with improved results. Research is ongoing to determine whether prenatal or postnatal interventions result in better MMC and CDH outcomes in both short- and long-term survival. The pharmacist can play a role in this patient population by providing counseling on medication management and dietary supplementation.
REFERENCES
1. Cohnstein J, Zuntz N. Untersuchungen über das blut, den kreislauf und die athmung beim Säugethier-Fötus. Archiv für die gesamte Physiologie des Menschen und der Tiere. 1884;34:173-233.
2. Liley AW. Intrauterine transfusion of foetus in haemolytic disease. Br Med J. 1963;2:1107-1109.
3. Maselli KM, Badillo A. Advances in fetal surgery. Ann Transl Med. 2016;4:394.
4. Canfield MA, Honein MA, Yuskiv N, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Res A Clin Mol Teratol. 2006;76:747-756.
5. Dukhovny S, Wilkins-Haug L. Open neural tube defects: risk factors, prenatal screening and diagnosis, and pregnancy management. UpToDate. Waltham, MA: UpToDate; 2018.
6. Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527-532.
7. Copp AJ, Adzick NS, Chitty LS, et al. Spina bifida. Nat Rev Dis Primers. 2015;1:15007.
8. Shurtleff DB, Luthy DA, Nyberg DA, et al. Meningomyelocele: management in utero and post natum. Ciba Found Symp. 1994;181:270-286.
9. Hunt GM. The median survival time in open spina bifida. Dev Med Child Neurol. 1997;39:568.
10. Manning SM, Jennings R, Madsen JR. Pathophysiology, prevention and potential treatment of neural tube defects. Ment Retard Dev Disabil Res Rev. 2000;6:6-14.
11. Korenromp MJ, van Gool JD, Bruinese HW, Kriek R. Early fetal movements in myelomeningocele. Lancet. 1986;1:917-918.
12. Sival DA, Begeer JH, Staal-Schreinemachers AL, et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum Dev. 1997;50:27-37.
13. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. New Engl J Med. 2011;364:993-1004.
14. Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet. 2004;364:1885-1895.
15. Adzick NS. Prospects for fetal surgery. Early Hum Dev. 2013;89:881-886.
16. Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31:183-188.
17. Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826-1831.
18. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131-137.
19. CDC. Folic acid: recommendations. www.cdc.gov/ncbddd/folicacid/recommendations.html. Accessed February 13, 2018.
20. Drugs that deplete: vitamin B9 (folic acid). www.stlukes-stl.com/health-content/medicine/33/000722.htm. Accessed March 28, 2018.
21. Tonks A, Wyldes M, Somerset DA, et al. Congenital malformations of the diaphragm: findings of the West Midlands Congenital Anomaly Register 1995 to 2000. Prenat Diagn. 2004;24:596-604.
22. Doyle NM, Lally KP. The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol. 2004;28:174-184.
23. Kardon G, Ackerman KG, McCulley DJ, et al. Congenital diaphragmatic hernias: from genes to mechanisms to therapies. Dis Model Mech. 2017;10:955-970.
24. Irish MS, Holm BA, Glick PL. Congenital diaphragmatic hernia. A historical review. Clin Perinatol. 1996;23:625-653.
25. Menon SC, Tani LY, Weng HY, et al. Clinical characteristics and outcomes of patients with cardiac defects and congenital diaphragmatic hernia. J Pediatr. 2013;162:114-119.e2.
26. Dillon PW, Cilley RE, Mauger D, et al. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:307-312.
27. Lusk LA, Wai KC, Moon-Grady AJ, et al. Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. J Pediatr. 2015;166:251-256.e1.
28. Keijzer R, Liu J, Deimling J, et al. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299-1306.29. UCSF Fetal Treatment Center. Congenital diaphragmatic hernia (CDH). https://fetus.ucsf.edu/cdh. Accessed March 27, 2018.
30. Derderian SC, Jayme CM, Cheng LS, et al. Mass effect alone may not explain pulmonary vascular pathology in severe congenital diaphragmatic hernia. Fetal Diagn Ther. 2016;39:117-124.
31. Graves CE, Harrison MR, Padilla BE. Minimally invasive fetal surgery. Clin Perinatol. 2017;44:729-751.
32. Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916-1924.
33. Al-Maary J, Eastwood MP, Russo FM, et al. Fetal tracheal occlusion for severe pulmonary hypoplasia in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of survival. Ann Surg. 2016;264:929-933.
34. Hedrick HL. Management of prenatally diagnosed congenital diaphragmatic hernia. Semin Pediatr Surg. 2013;22:37-43.
35. Deprest J, De Coppi P. Antenatal management of isolated congenital diaphragmatic hernia today and tomorrow: ongoing collaborative research and development. J Pediatr Surg. 2012;47:282-290.
36. Clugston RD, Klattig J, Englert T, et al. Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am J Pathol. 2006;169:1541-1549.
37. National Institutes of Health Office of Dietary Supplements. Vitamin A: fact sheet for consumers. https://ods.od.nih.gov/factsheets/VitaminA-Consumer. Accessed February 22, 2018.
38. National Institutes of Health Office of Dietary Supplements. Vitamin A: fact sheet for health professionals. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional. Accessed February 23, 2018.
39. Xeropthalmia. UpToDate. Waltham, MA: UpToDate; 2018.
To comment on this article, contact rdavidson@uspharmacist.com.






